Bye-bye, bleaching!
Recent years have seen a rapid evolution of live-cell research on the molecular scale as new microscopy techniques have constantly increased the achievable resolution in space and time. But it is not only the development of new microscopy concepts, better optics and detectors fueling the field’s progress. Fluorescent labeling strategies have become more and more sophisticated and offer ever-new options to improve microscopic imaging, such as exchangeable HaloTag ligands that put an end to photobleaching for good.
Hello, exchangable HaloTag®!
Confocal and superresolution microscopy rely on the specific labeling of proteins of interest with a fluorescent tag. The classic approach to this end is immunofluorescence, where antibodies carrying a fluorescent label recognize and selectively bind to target proteins (you may learn more about immunofluorescence here). Thanks to its versatility, this strategy is still the most popular. However, there are drawbacks, one of them being that antibody staining does not work in living cells. But the recent development of nanobodies opens new ways here and adds to the strategy’s potential in particular in superresolution microscopy (again, another article in our knowledge base will tell you more).
And there are other strategies to make your sample light up under the microscope which come along with distinct advantages, like co-expression of a fluorescent protein or fluorescent labeling via click-chemistry.
The HaloTag® system – a true glue
A comparably new option is the HaloTag®.1 The HaloTag® is a small protein derived from the bacterial enzyme halokane dehalogenase.2,3 It can be fused to a protein of interest by genetic engineering and binds synthetic ligands. These HaloTag® ligands are key players in the labeling process. They consist of a linker and a functional group (we will come to what this is in a moment). Once the ligand comes into the vicinity of the HaloTag® protein, a covalent bond – i.e. a very stable chemical connection – is formed between the protein and the linker: they stick together as if glued, permanently uniting the protein with the ligand’s functional group. And this functional group can be many things, depending on the experimental setting. One thing the functional group can be is a fluorophore.
The HaloTag® thus allows to precisely target proteins of interest with fluorescent labels, opening the door to a wide range of applications in (superresolution) microscopy. In particular live-cell imaging benefits from the use of HaloTag® ligands carrying organic fluorophores as these can easily enter living cells and don’t harm them. But also in fixed cells and other in vitro systems the HaloTag® demonstrates its power and versatility as the stability of the protein-ligand gluing guarantees good staining results even under challenging conditions.2 Last but not least, the HaloTag® system comes along with minimal background fluorescence and an excellent signal-to-noise ratio, both criteria decisive for microscopic imaging.
So with the HaloTag® we have a super-stable protein-ligand complex, strong imaging contrast, and can do live-cell imaging. All good, end of story? Not quite… as every coin has a flip side.
Bleached stays bleached?
For fluorophores, this is photobleaching: Fluorophores exposed to light will bleach at some point. When quasi-permanently bound to their target proteins and exposed to a lot of very powerful and focused light – as is usually the case in fluorescence microscopy – this point will be reached quite soon and certainly sooner than any microscopist could wish. And once this point is reached, there is no way to restore fluorescence – pretty much the same as a poster exposed to sunlight for a long time will lose its color and the only option to restore it to original brightness is to replace the poster with a fresh version. With covalently coupled fluorophores, this is not possible at any rate. Bleached stays bleached.
There are, however, ways of fluorophore coupling that are not permanent – more the kiss-and-run site than the glue-site, so to speak. One example is DNA-point accumulation for imaging in nanoscale topography (PAINT)4: It uses short stretches of DNA as ligands. The DNA stretches specifically but transiently bind to their targets – which are DNA sequence tags on the proteins of interest –, i.e. they bind and unbind at a specific rate. The advantage in terms of photobleaching is clear: As fluorophores at target proteins are constantly replaced by “fresh” ones from the virtually unlimited reservoir in the surrounding buffer, bleaching is no longer an issue.
Should I stay or should I go?
Which brings us back to the HaloTag®: Scientists were not satisfied (when are they ever?) with the classical glue-type HaloTag®’s limitations and were looking for ways to expand its applicability.5 So they chemically engineered the HaloTag® ligand, replacing the chloride atom in the linker with a sulfur-containing reactive group. This seemingly miniature change brought about a major change in the molecule’s chemical behavior: The manipulated linker still fits into the reactive pocket of the HaloTag® protein but the chemical reaction forming the covalent bond can no longer take place: The HaloTag® ligand binds and unbinds only transiently, just like the DNA in DNA-PAINT. It has become exchangeable and we now call it HaloX®.

Schematic illustration of the conventional covalent HaloTag® labeling with a fluorescent HaloTag® ligand compared to novel non-covalent HaloTag® labeling with exchangeable fluorescent HaloTag® ligands (HaloX®).
Note that only the ligand is changed but not the protein. This means that HaloX® is easily applicable: Researchers previously using the HaloTag® system can continue with their established HaloTag®-coupled proteins: Just use the new HaloX® ligands and go ahead!
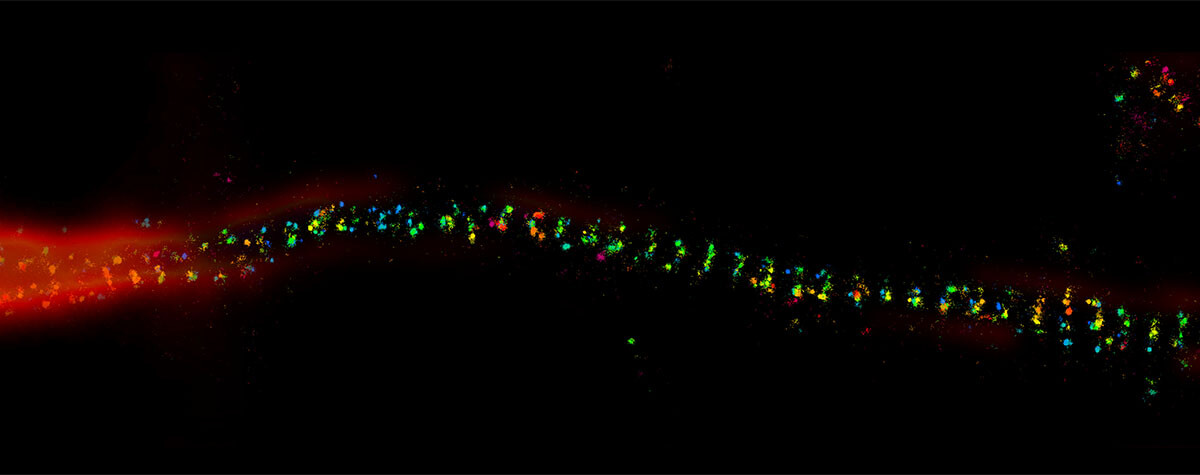
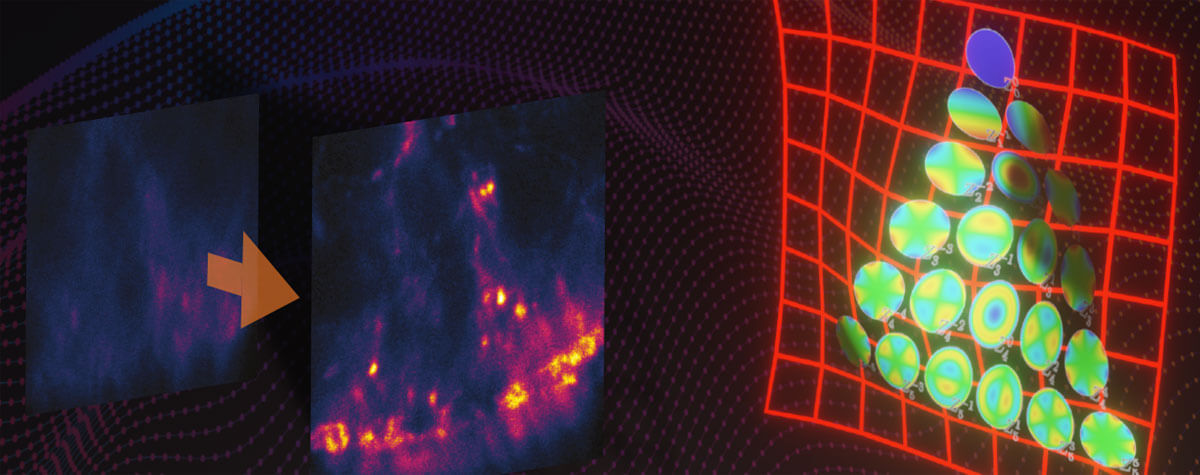
HaloX® is an alternative to DNA-coupled labeling in PAINT and also expands opportunities for MINFLUX microscopy: The MINFLUX concept relies on fluorophores to switch between light and dark states to avoid signal overlap and to achieve its exceptional spatial resolution and high localization precision.
Binding switches on the light
Generally, such stochastic light-dark-switching can be achieved by intrinsic characteristics of fluorophores such as conformational changes that either suppress or allow fluorescence to occur. With HaloX®, like with other exchangeable dyes, it is no longer necessary to work with stochastically switching fluorophores. The light-dark-switch is achieved by the binding-unbinding of the fluorophore to its target, like in DNA-PAINT for MINFLUX6: While freely diffusing, the coupled fluorescent labels remain “dark”. Upon transient binding to a target protein, they light up, thus delivering photons from a defined position. This behavior is called fluorogenic.
Fluorogenicity is a property generally required for exchangeable ligands, not only in the context of MINFLUX: In classical staining strategies such as antibody labeling, residual and unbound fluorophores are washed away before imaging. Working with fluorophores coupled to exchangeable ligands, in contrast, requires imaging to take place in the presence of fluorophores freely diffusing in the buffer as a reservoir to constantly replace those bound to target proteins. If the fluorophores were not fluorogenic, they would be excitable also in their unbound state, resulting in extremely high background staining.
The potential of HaloX® is not yet exhausted. Currently, it is restricted to one-color imaging as a second color requires the HaloTag® protein to be modified. However, it has already been shown that suitable combinations of modified HaloTag® protein and linker are possible.5 This will facilitate two-color imaging using two different exchangeable HaloTag® variants and will extend the system’s applicability even more.
1 Urh M, Rosenberg M. HaloTag, a Platform Technology for Protein Analysis. Curr Chem Genomics. 2012;6:72-8. doi: 10.2174/1875397301206010072. Epub 2012 Dec 5. PMID: 23213345; PMCID: PMC3480824.
2 England, Christopher G., Haiming Luo, and Weibo Cai. “HaloTag technology: a versatile platform for biomedical applications.” Bioconjugate chemistry 26.6 (2015): 975-986.
3 Wilhelm, Jonas, et al. “Kinetic and structural characterization of the self-labeling protein tags HaloTag7, SNAP-tag, and CLIP-tag.” Biochemistry 60.33 (2021): 2560-2575.
4 Sharonov, Alexey & Hochstrasser, Robin M. “Wide-field subdiffraction imaging by accumulated binding of diffusing probes.” PNAS 103 (2006): 18911-18916.
5 Kompa, Julian, et al. “Exchangeable halotag ligands for super-resolution fluorescence microscopy.” Journal of the American Chemical Society 145.5 (2023): 3075-3083.
6 Ostersehlt, Lynn M., et al. “DNA-paint minflux nanoscopy.” Nature Methods 19.9 (2022): 1072-1075.
HaloTag® is registered trademark of Promega Corporation
HaloX® is registered trademark of Spirochrome AG. This product is covered by one or more license from Spirochrome AG and, is intended for Research Use Only (RUO).