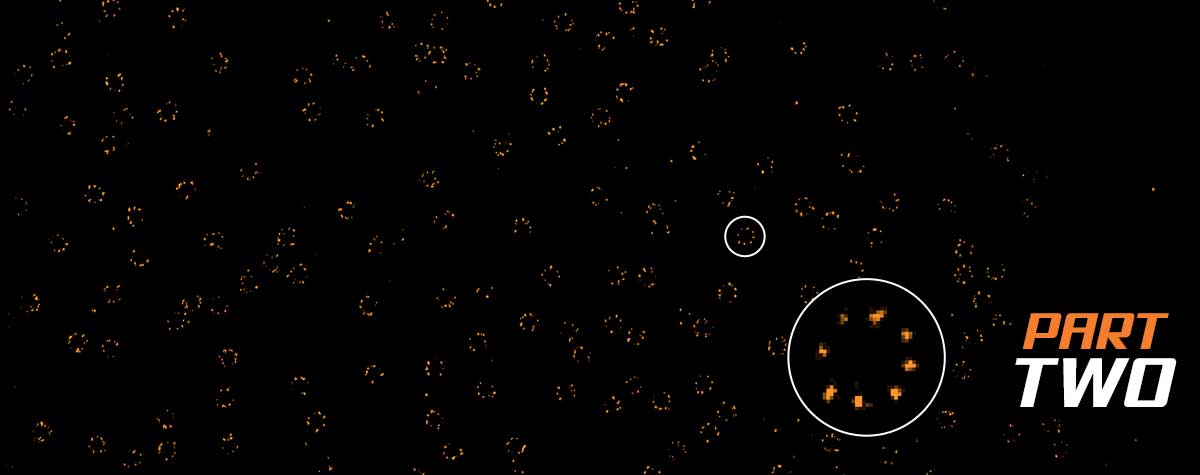
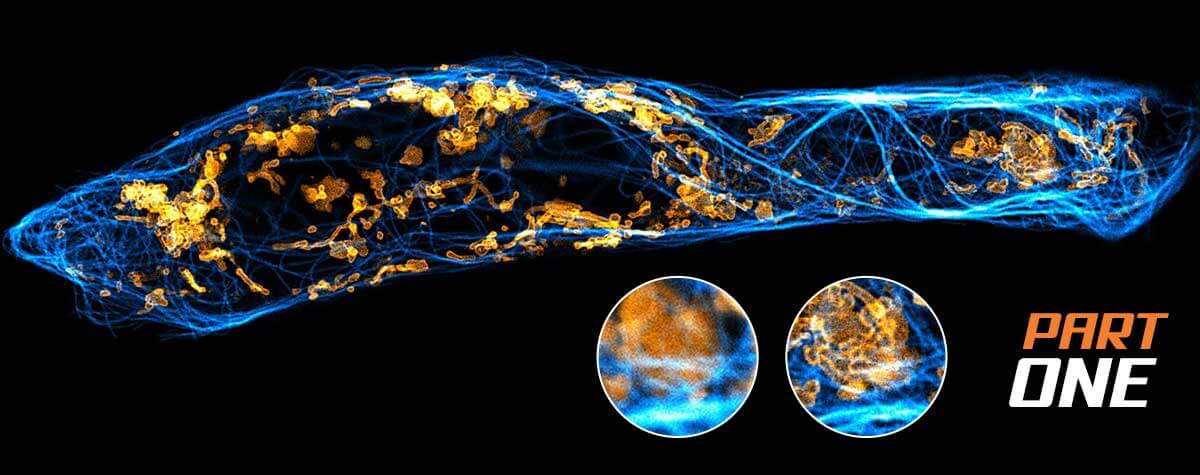
The price of a STED microscope
Biological research at the cellular and molecular level seems almost unthinkable without a microscope. Microscopy methods have opened new avenues to examine structure and function within the cell and publishing expectations almost invariably call upon these to corroborate findings. Platform providers have also responded to demand. Today’s research microscopes are increasingly powerful, modular, and combinatorial. There’s a lot of options out there.
what’s the value?
Thus, being in the market for a high-quality, high-performance research microscope can feel like navigating a minefield. There are numerous types, models, and makes of microscopes to consider. The language of listed features often uses enigmatic brand names that obscure comparability, especially for buyers beginning to delve into available technologies. And then there’s your budget. It’s not helpful to size it up to the generic and broad price ranges accessible without a quote.
Prices for a decent but very basic confocal microscope start at around 100,000 US dollars, while list prices for high-end confocal systems sometimes reach a million dollars. Including superresolution features like MINFLUX, PALM/STORM, or STED typically adds another few 100,000 dollars to the price each, so that the required spending for the most sophisticated superresolution microscope can be well over a million. However, consider that an electron microscope with a similar resolution as MINFLUX can cost many times more.
Be aware that taking these price ranges at face value is misleading. You can’t really compare an entry-level transmission electron microscope to, for example, a high-end MINFLUX microscope. Comparing price ranges may help you exclude some platform categories. A category may exceed your budget. The type of instrument may not match your microscopy needs (do you really need an electron microscope?). The size of objects you wish to visualize may require a resolution beyond the diffraction limit.
But while the price of a microscope is unquestionably a deal-breaker for its purchase, a more helpful criterion is its value. So, once you have narrowed down your options, think about the long-term nature of your research. Specifically, compare and contrast three aspects: resolution, flexibility, and ease of use.
Do you anticipate needing greater resolution?
The higher the resolution you need, the more limited your choice of microscopes. To visualize objects separated by less than 20 nm, your choices are MINFLUX (or a similar non-commercial platform) or electron microscopy. The price tag of these instruments reflects the complexity of their underlying technology (which often correlates with complex sample and instrument handling). Nevertheless, MINFLUX offers a comparative edge if you seek to visualize living cells and are looking for high temporal resolution.
Temporal resolution, you ask? Yup. If you are only interested in snapshots of cellular processes, you don’t need good temporal resolution. You’ll be taking a single image. If, however, you are interested in the dynamic changes that occur in a cell, investing in a fast microscope can generate savings in the long-run. An interactive graph in the article “Superresolution for biology: when size, time, and context matter” lets you examine the tradeoffs and coverage of spatial and temporal resolution for a range of platforms, from widefield to expansion and scanning electron microscopy.
Is one microscopy technology enough?
Most microscopes today are built to offer modular functionality, allowing you to combine different microscopy methodologies into one instrument. Naturally, that modularity comes with careful engineering that ensures robust, seamless, and harmonized operation of each module regardless of when or how you use it. Add more modules to your microscope, expect the price to go up. The combination of modules is also finite and may entail compromises for other dimensions. Keep these tradeoffs in mind as you evaluate how you will meet research needs. Nevertheless, an investment in a scope that allows you to explore more than one microscopy methodology, although expensive in the short-term, will likely save money in the long-run.
Who should use the microscope and how will they learn to use it?
Ease of use is often a “last thought” criterion in the purchase of advanced equipment. And yet, it is pivotal to the return on your investment. An advanced microscope in a lab is meant to produce data. That means that the instrument should be used as extensively and by as many people as possible, preferably without tying up your time. That simply won’t happen if handling is complicated and fastidious.
Value goes far beyond financials
Some publications outline ways to upgrade an existing microscope to new performance levels or to build your own platform for a fraction of the price of a commercial instrument.1,2 With the right expertise in laser and optics technology, such DIY platforms are an option. However, your time and the progress of your research are as relevant to your success as your budget. Consider the time you need to set up and troubleshoot such a solution. And will everyone in your lab be able to use it? How will you train operators? Furthermore, how will you handle instrument downtime when repairs or improvements are needed? All these factors influence your return on investment.
Flexibility, power, and ease of use are the key to a microscope that serves for many years and across many projects. Bearing all that in mind, a slightly higher price point may in fact be better value.
1 Ma et al. 2017. Nature. doi: https://doi.org/10.1038/s41598-017-01606-6
2 Danial et al. 2022. Nature Protocols. doi: https://doi.org/10.1038/s41596-022-00730-6