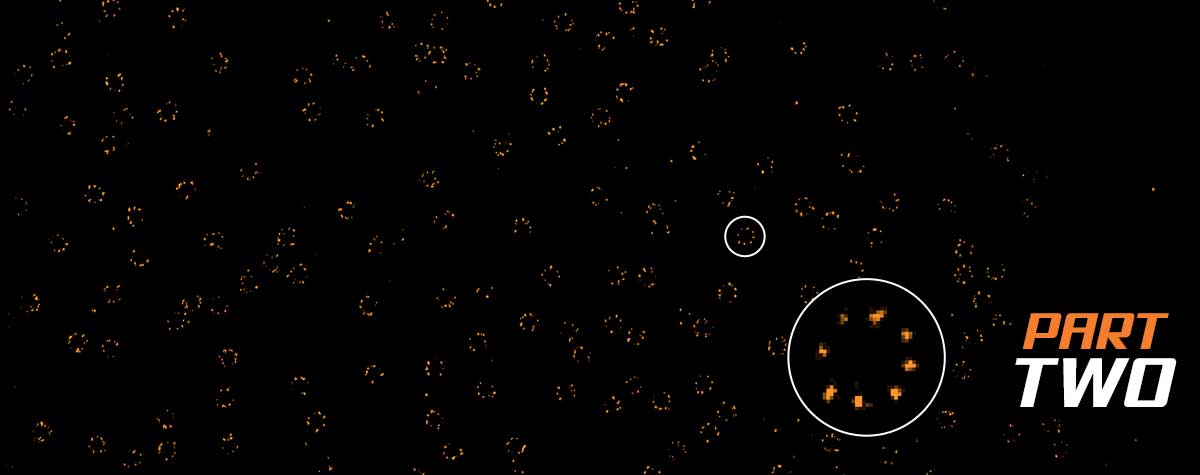
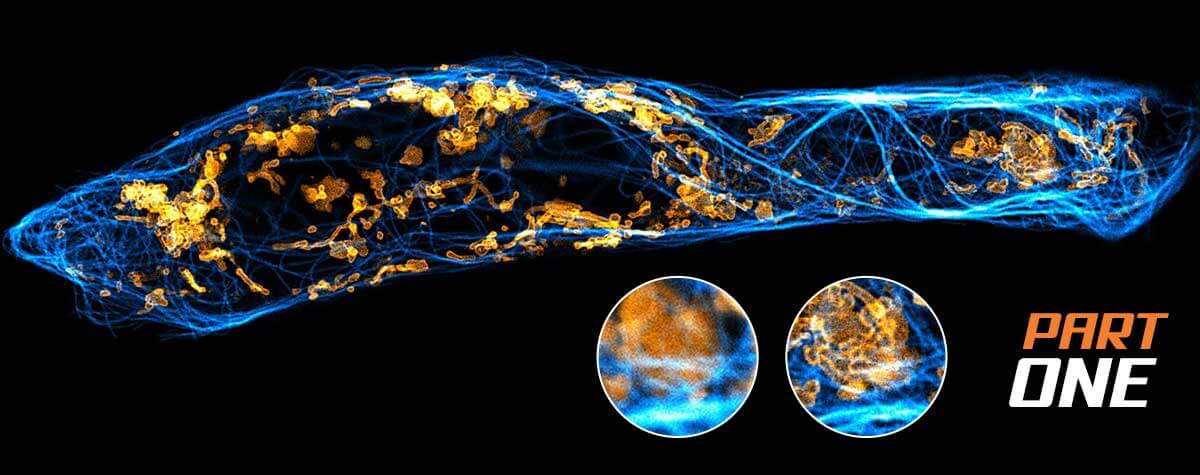
How PSF width and photon number
Photon numbers from the emitting fluorophore. Width of the PSF. How do they impact the resolution of a microscope? Here’s a simple graphic that lays out those effects.
impact resolution
In other articles on resolution – “What is resolution” and “How to measure resolution” – we talk about how a wide point spread function (PSF) reduces the resolution of a microscope as its blur makes two points indistinguishable in the resulting image. The physical width of the PSF cannot be smaller than the diffraction limit but superresolution technologies circumvent that boundary to generate an effective PSF width down to 20 nm or less. One workaround strategy, relies heavily on high photon flux from the fluorophore to localize it based on a likelihood fit to the shape of its PSF. That is, more photons per emitter is good. Another workaround strategy narrows the width of the PSF by restricting the area where a fluorophore can emit. A narrower full width at half maximum (FWHM) of the PSF is good.
The interaction between these two parameters – FWHM and photon number per emitter – is worth a closer look.
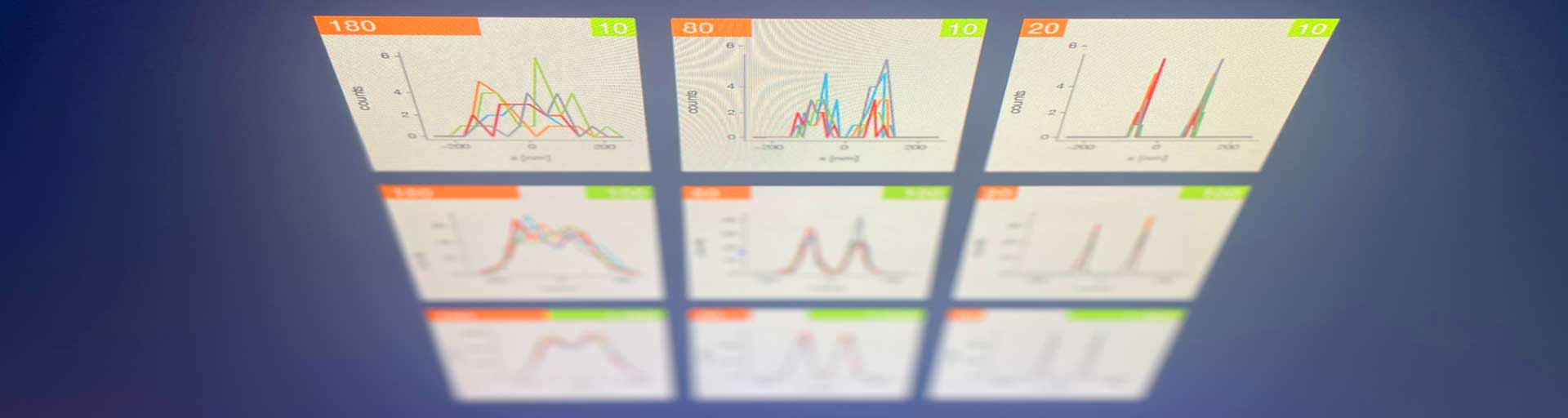
Take two emitters separated by a distance of 150 nm and image them multiple times. Now, let’s look at what happens to the intensity profiles of their PSFs when we alter the FWHM and the number of photons emitted by the fluorophores. In the graphic below, each colored line is a separate imaging run.
Line profiles, two emitters at 150 nm distance
![]() Full width at half maximum (FWHM) [nm]
Full width at half maximum (FWHM) [nm]
![]() Photons per emitter (PPE)
Photons per emitter (PPE)
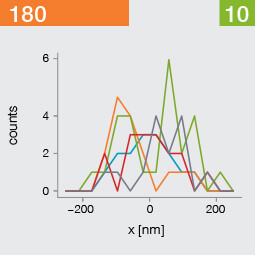
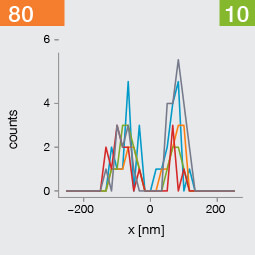
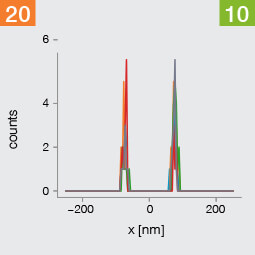
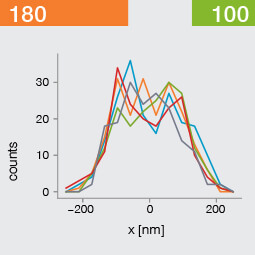
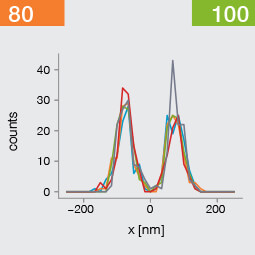
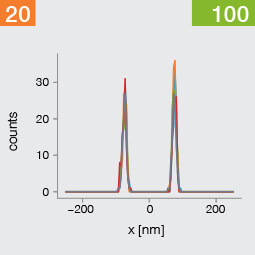
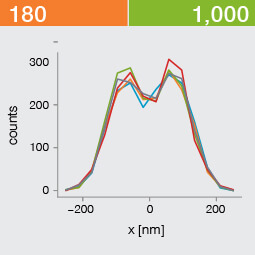
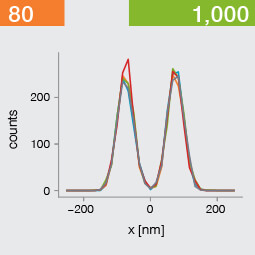
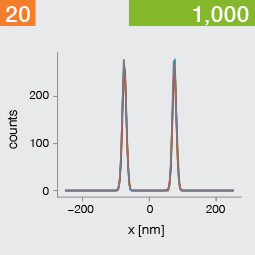
A low photon emission rate results in a noisy PSF, dramatically limiting how well the two emitters can be discriminated. That noise, however, becomes less relevant in separating the two PSF when you have a narrower FWHM. Look at the graphic from left to right. What is surprising is that for a small FWHM, only a handful of photons are sufficient to clearly distinguish the two PSFs. Perhaps an alternative: The narrow FWHM teases apart the signals of the two fluorophores despite the low and inconsistent detection of photons between runs.
Resolution also improves as the number of photons detected from each emitting fluorophore rises. Look at the graphic from top to bottom. The individual measurements become more consistent to reveal a distinct dip between the two PSF intensity profiles. It can be clearly seen that if the FWHM is too large, even a higher number of photons than in our example will no longer improve the resolution.
Key, however, is that the discrimination power of the microscope improves more readily with the narrower FWHM, even with lower photon flux from the fluorophores. These are two levers at your disposal to reach higher resolution. Which will give you greater access to the nanoscale world? As so often in life, it depends!
One thing is clear, however: the narrower the FWHM, the fewer photons are needed for a high-resolution image.