Sample gallery
Fluorescence imaging, whether at confocal, STED or MINFLUX resolution, guarantees unique insights into the function and structure of life at the molecular level. Besides the scientific information content, some sample portraits provide simply beautiful images. Enjoy browsing our sample gallery.
the fine art of science
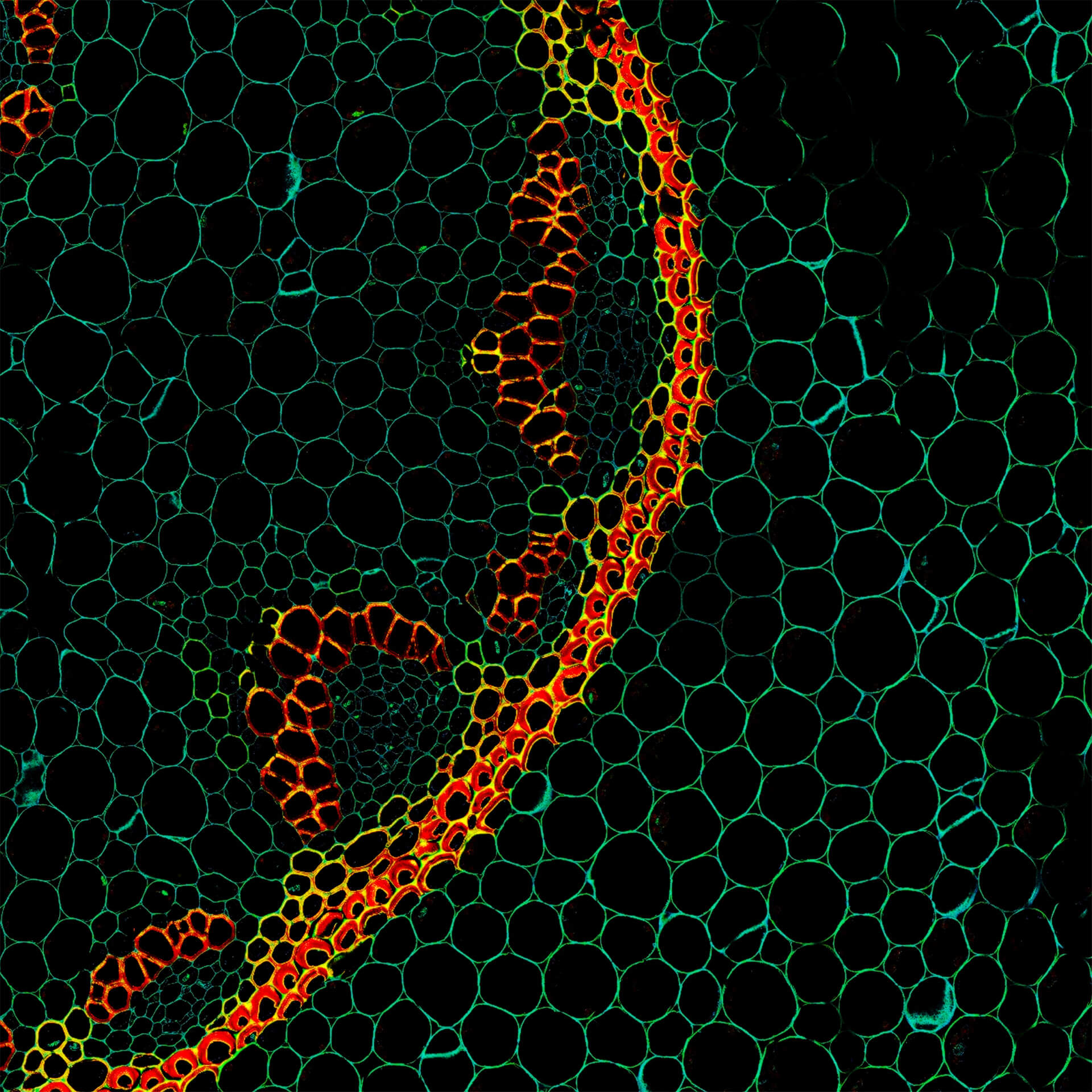
Description
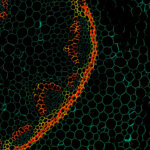
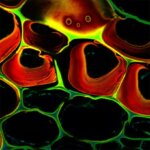
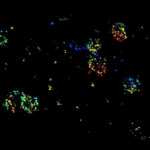
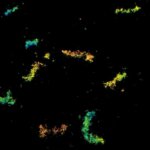
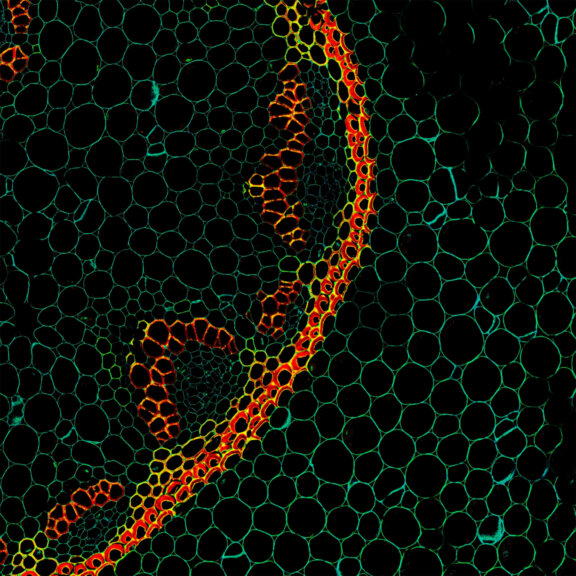
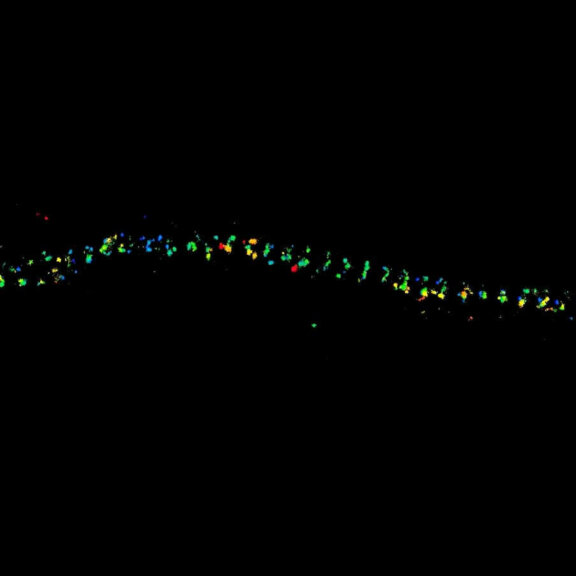
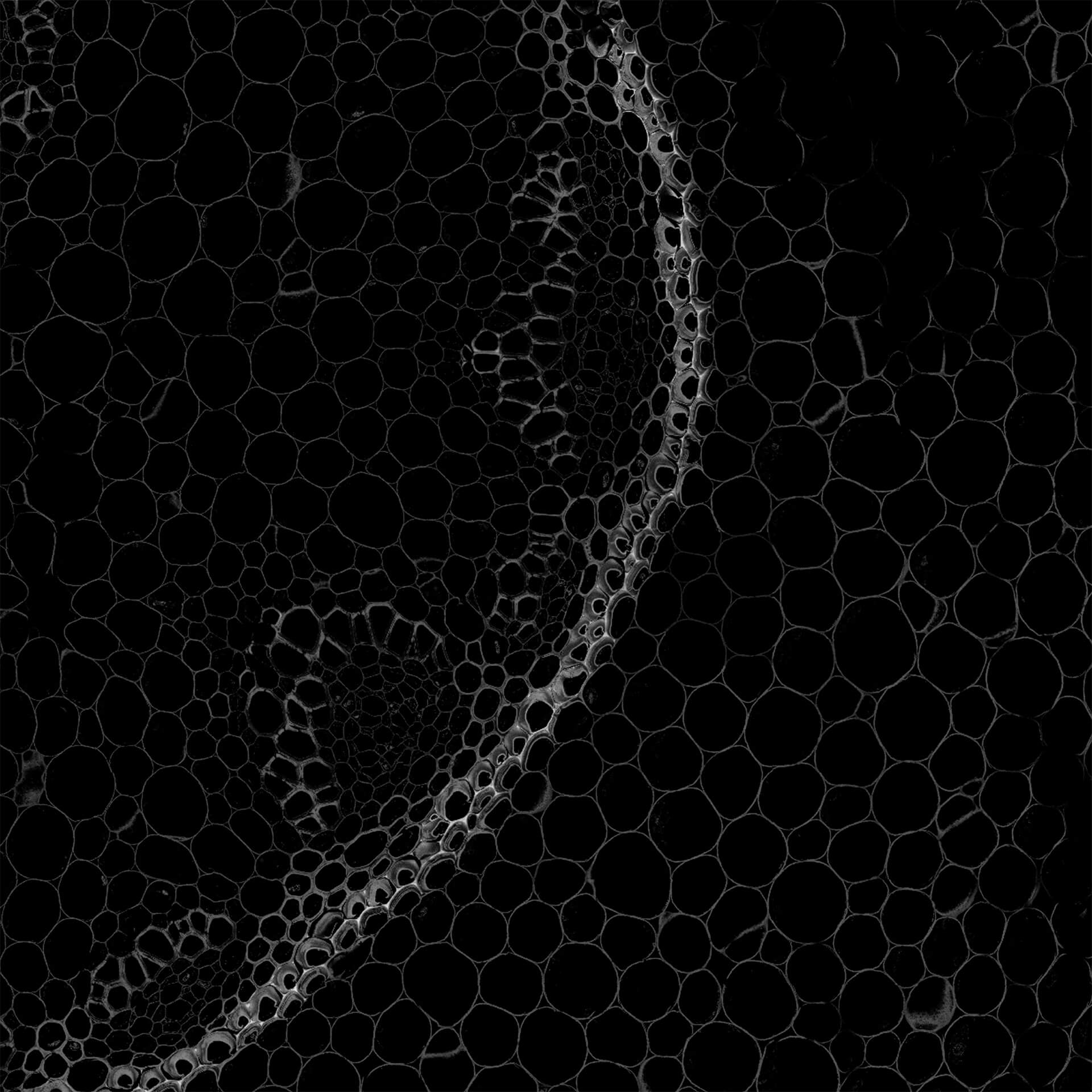
Autofluorescence in a plant stem cross section of Convallaria recorded with FACILITY. A large area, 10 by 10 tiles, each 125 µm by 125 µm, was imaged and stitched with image tiling in Fiji for ImageJ. This is combined with TIMEBOW lifetime imaging to show the differences in fluorescence lifetime due to the type of autofluorescent molecule and their nano-environment.
Modules:
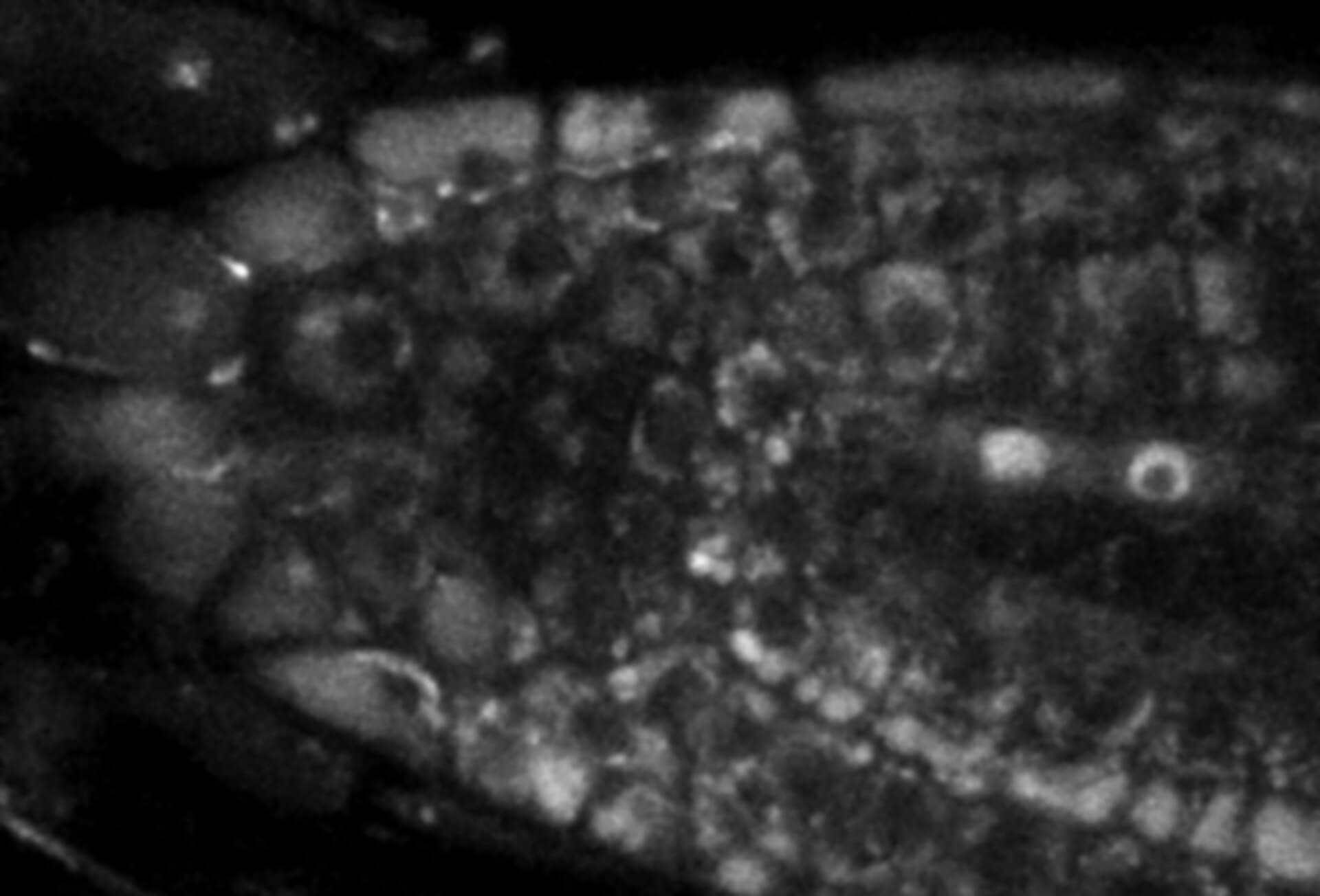
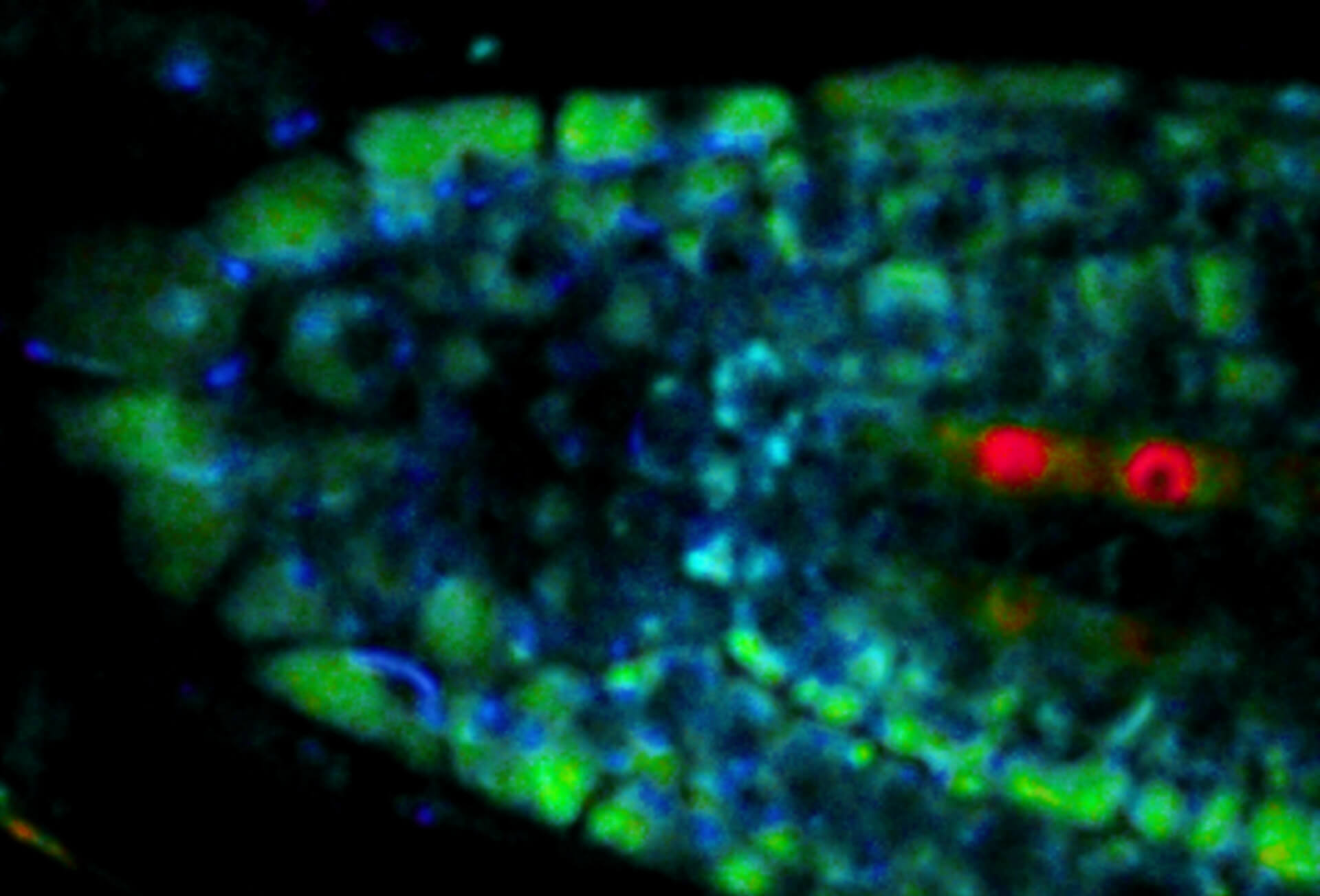
Description
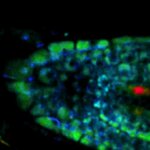
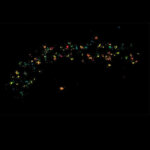
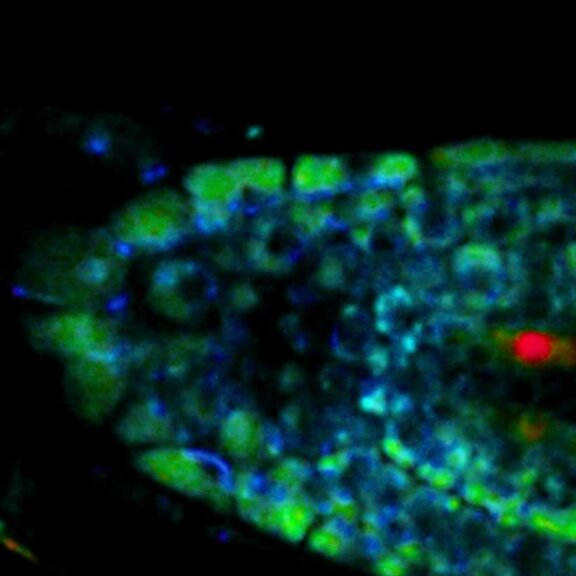
Live-cell sample of an Arabidopsis root tip suspended in water recorded with FACILITY. A subset of cells expresses a YFP construct. Lifetime imaging with TIMEBOW shows shifts in YFP fluorescence lifetime caused by the proteins nano-environment.
We thank Dr. Fábián Attila and Soós Vilmos (ATK, Brunszvik, HU) for providing this sample. Contact: soos.vilmos@atk.hu.
Modules:
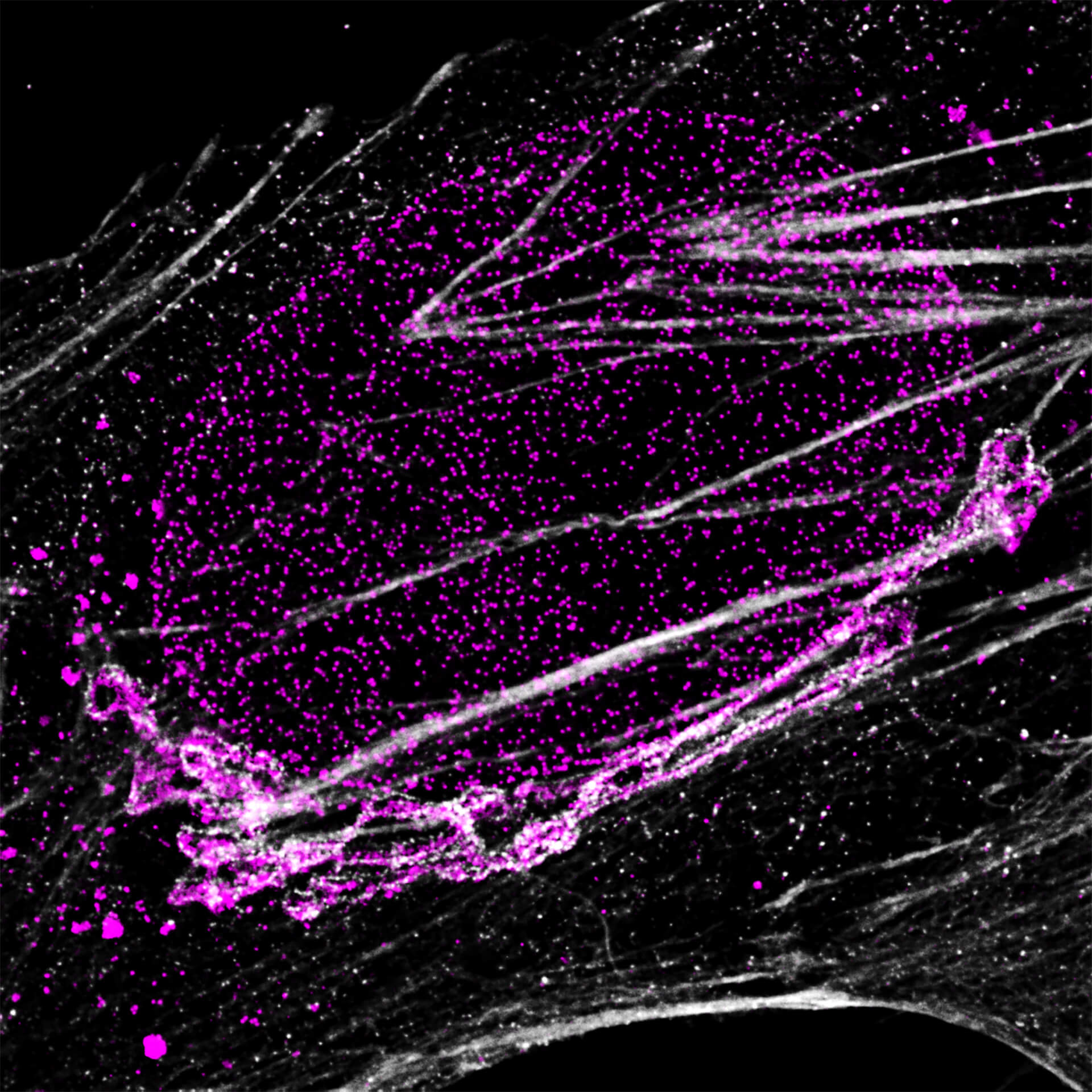
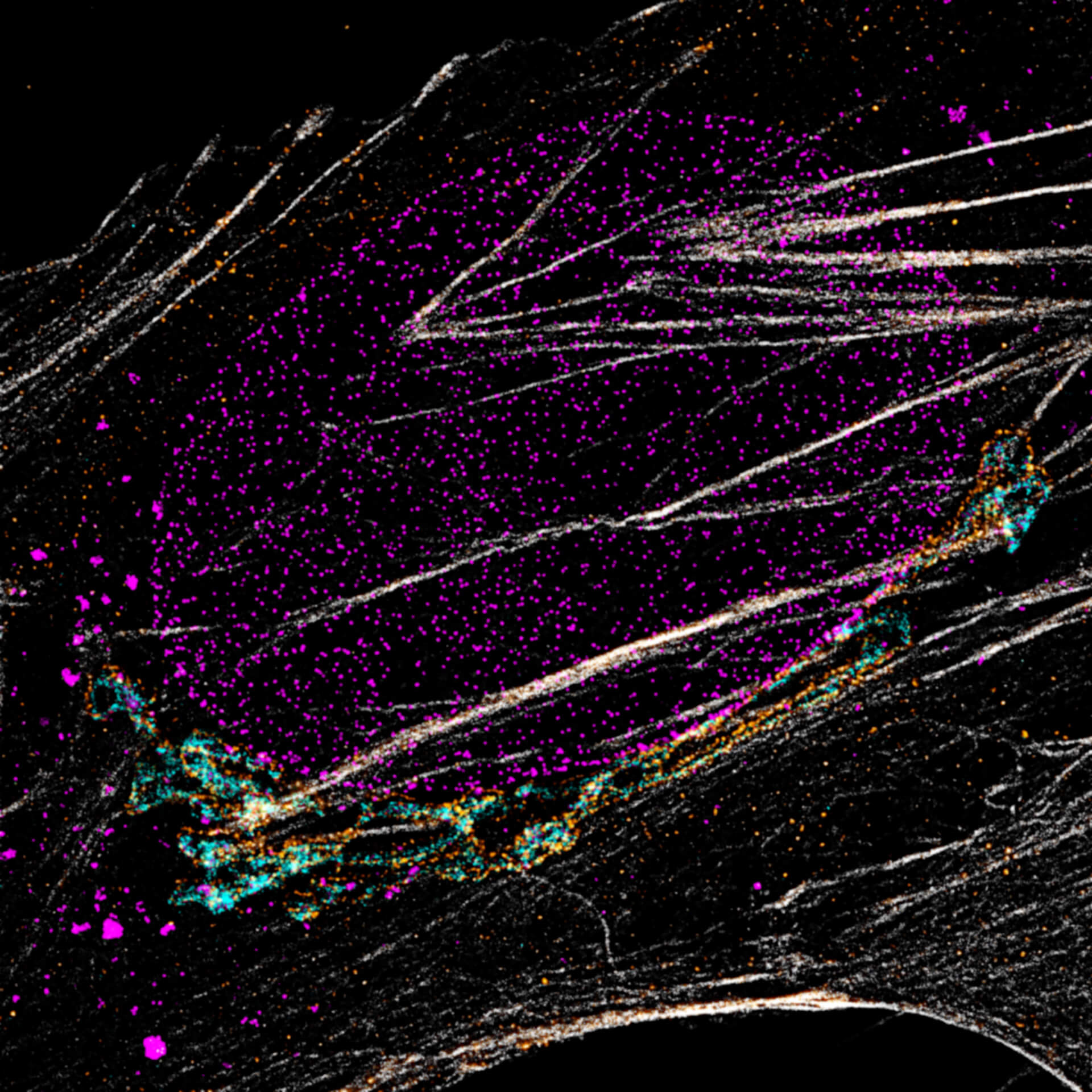
Description
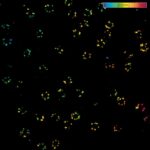
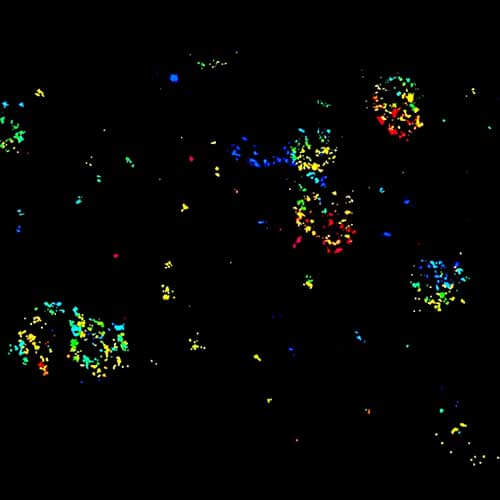
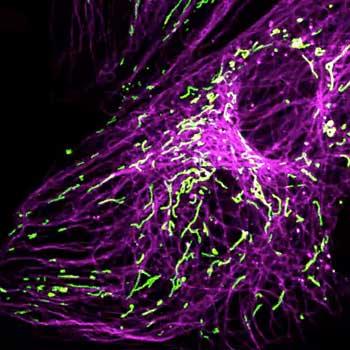
Four-color staining of fixed mammalian cells recorded with FACILITY showing nuclear pores (NUP98, STAR RED), Golgi membrane (GM130, STAR 635), Golgi medial rims (Giantin, STAR ORANGE) and actin (STAR 580 phalloidin). Shown left, just using spectral information, STAR RED and STAR 635 (magenta) cannot be separated, because of their similar excitation properties. The same goes for STAR ORANGE and STAR 580 (grey).
With the lifetime information collected by TIMEBOW, these dyes can be distinguished, turning an image with two recording channels into a four-color image. To achieve superresolution only one STED laser at 775 nm was used.
All utilized dyes are manufactured by abberior.
Modules:
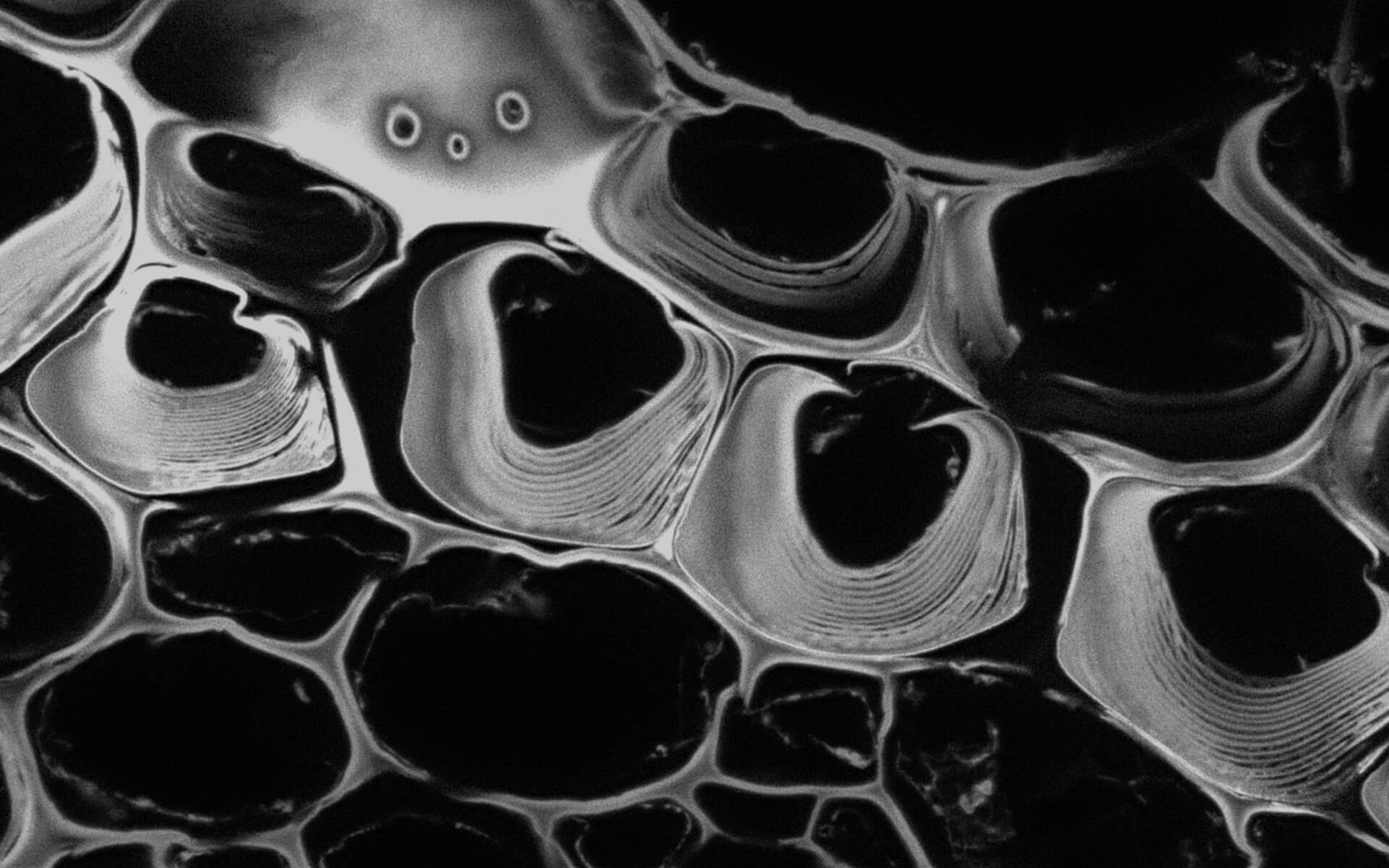
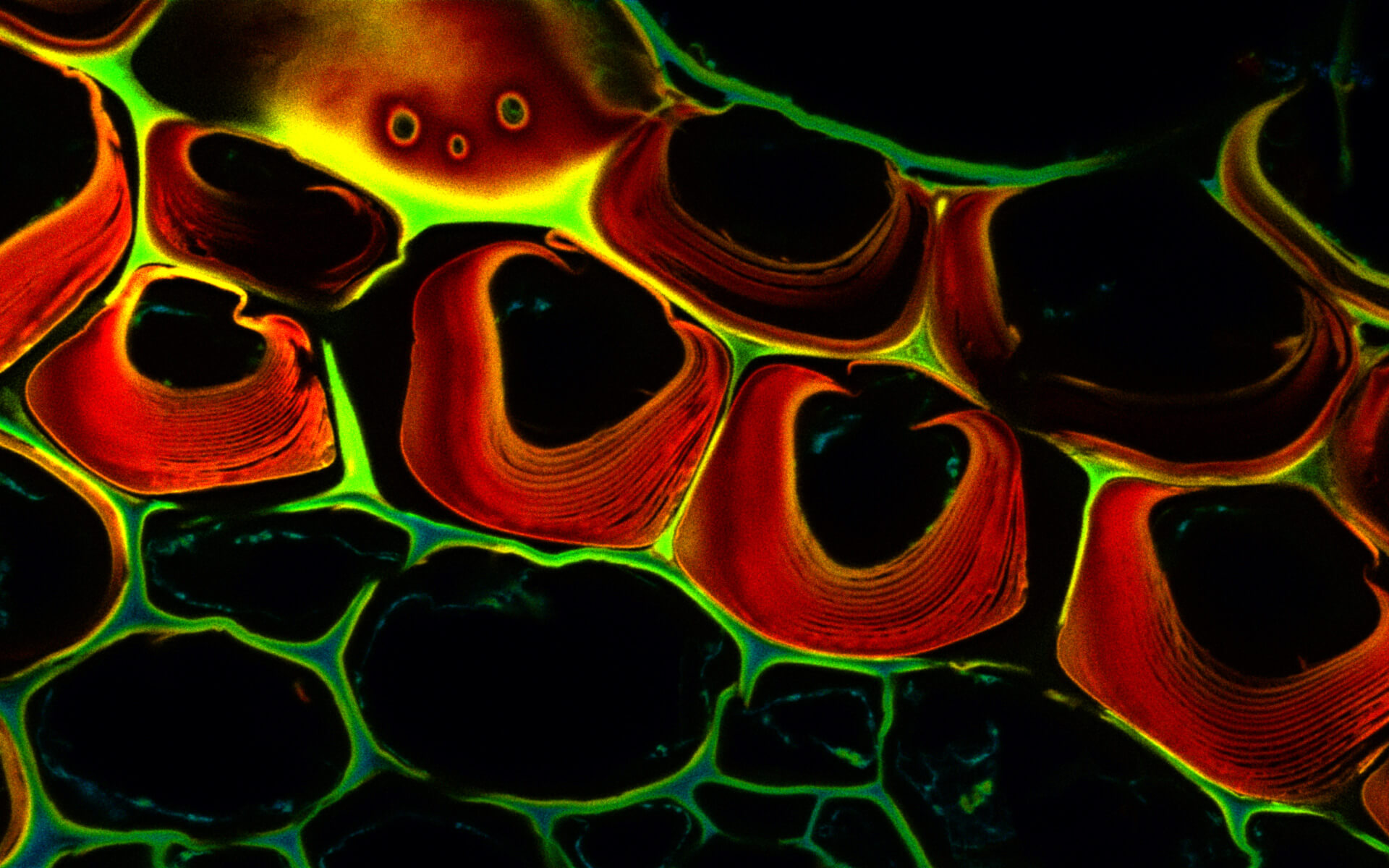
Description
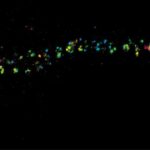
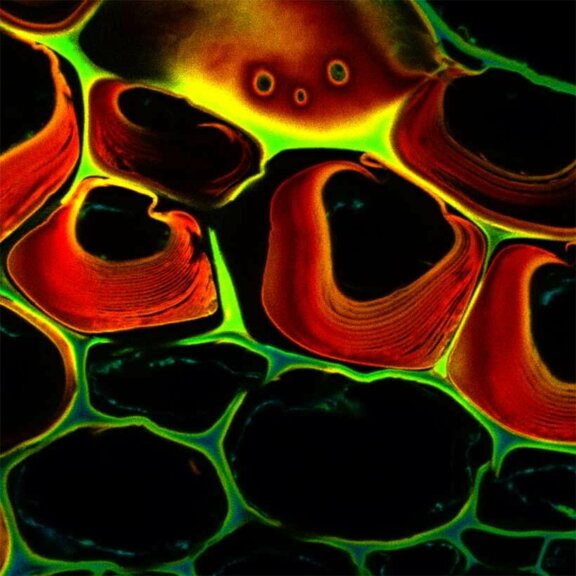
Autofluorescence in a plant stem cross section of Convallaria recorded with FACILITY. Image quality is improved by removing the background through MATRIX detection. This is combined with TIMEBOW lifetime imaging to show the differences in fluorescence lifetime due to the type of autofluorescent molecule and their nano-environment.
Modules:


Description
TIMEBOW STED vs MATRIX + TIMEBOW STED
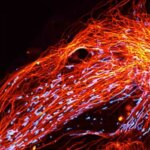
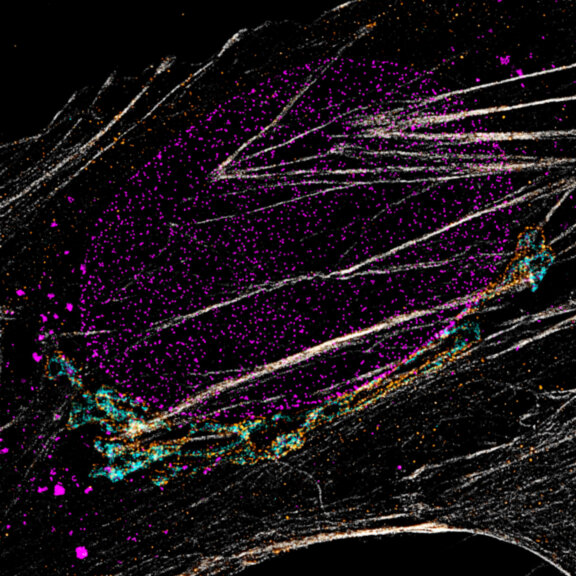
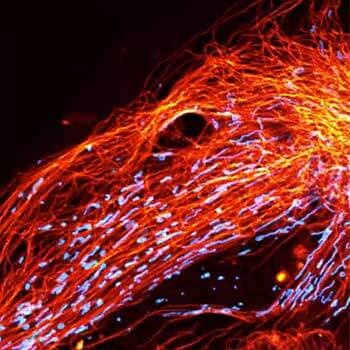
Actin in microvilli of CaCo2 cells recorded with FACILITY. Fixed cells were stained with abberior STAR RED phalloidin. Imaging of the intense-background sample is optimized by MATRIX detection by directly subtracting unfocussed data. To boost resolution TIMEBOW is used, keeping STED laser power low. Lifetime information enables the selective exclusion of STED-affected emission at the rim of each measurement.
We thank Prof. Dorothee Günzel and Jörg Piontek (Charité, Berlin, DE) for providing this sample.
Modules:

Description
Confocal vs MATRIX + TIMEBOW STED
Actin in microvilli of CaCo2 cells recorded with FACILITY. Fixed cells were stained with abberior STAR RED phalloidin. Imaging of the intense-background sample is optimized by MATRIX detection by directly subtracting unfocussed data. To boost resolution TIMEBOW is used, keeping STED laser power low. Lifetime information enables the selective exclusion of STED-affected emission at the rim of each measurement.
We thank Prof. Dorothee Günzel and Jörg Piontek (Charité, Berlin, DE) for providing this sample.
Modules:
Description
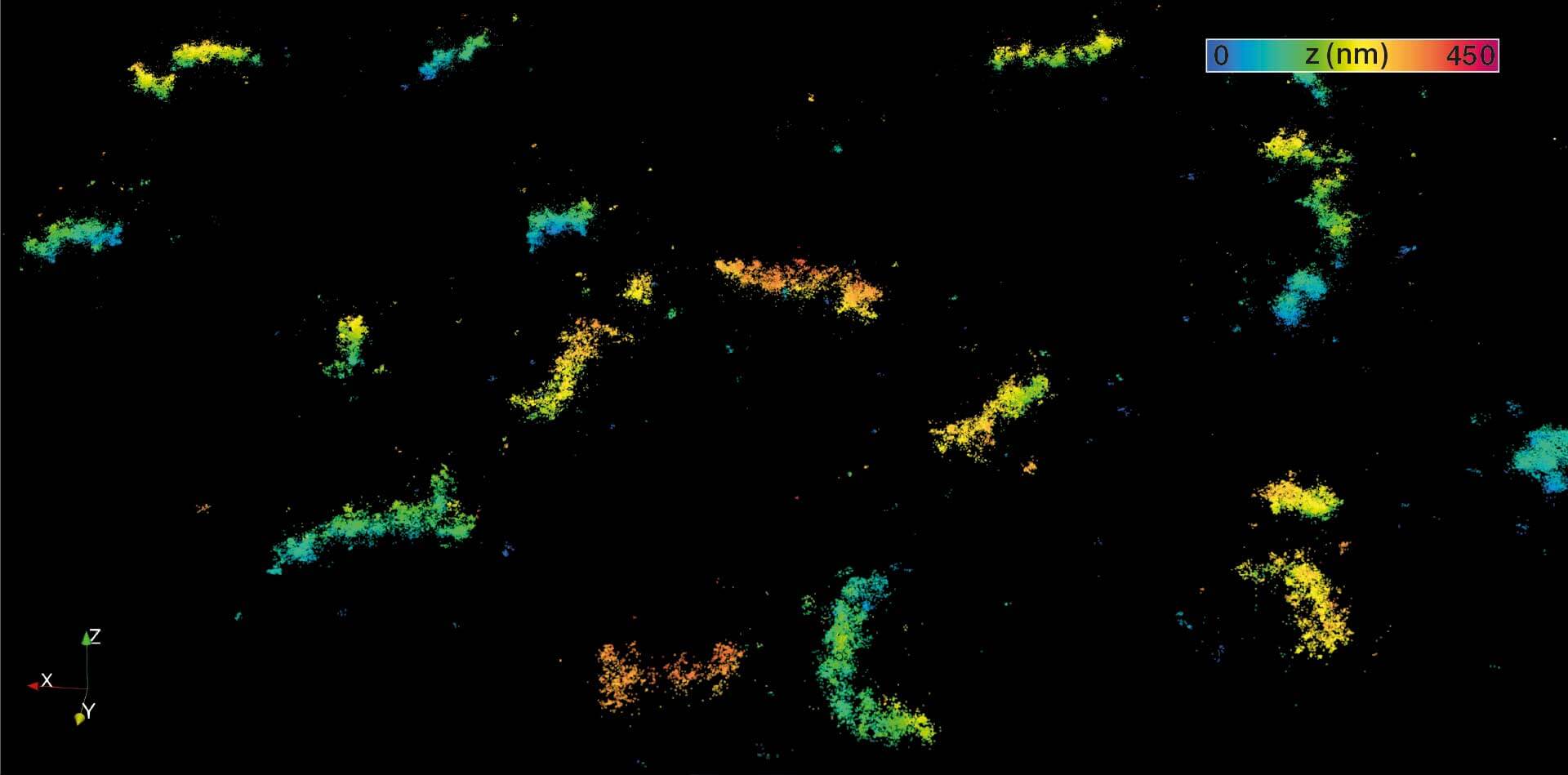
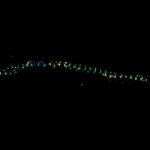
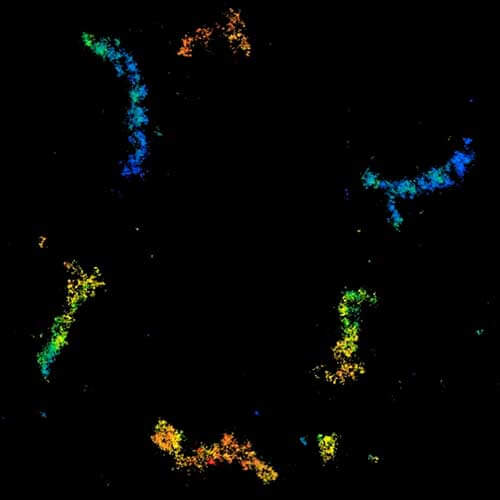
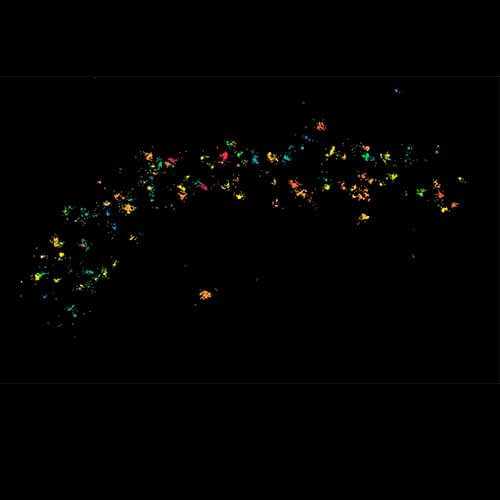
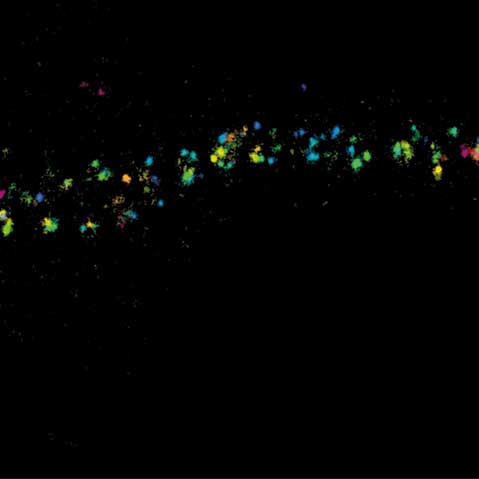
MINFLUX 3D imaging of βII spectrin in a primary hippocampal neuron labeled with Alexa Fluor 647 by indirect immunofluorescence. The axial coordinate is color-coded. Please note the periodic arrangement of spectrin along the axon.
Description
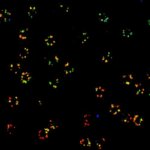
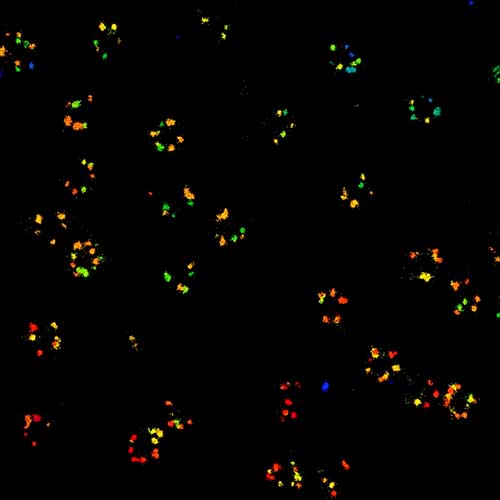
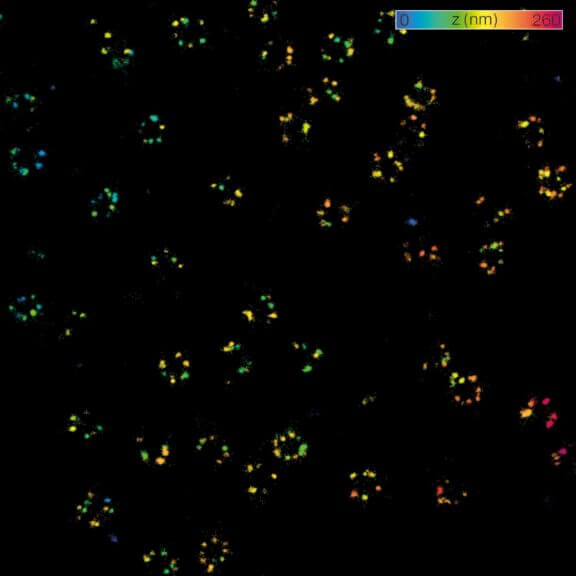
MINFLUX 3D nanoscopy of nuclear pore complex subunits. 3D MINFLUX facilitates 3D imaging at molecular scales. In the axial direction localization, we reach precisions of approx. 2.5 nm in raw localization data. Labeling of NUP96-SNAP was performed using SNAP-Alexa Fluor 647.
Description
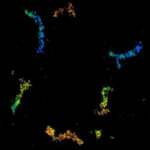
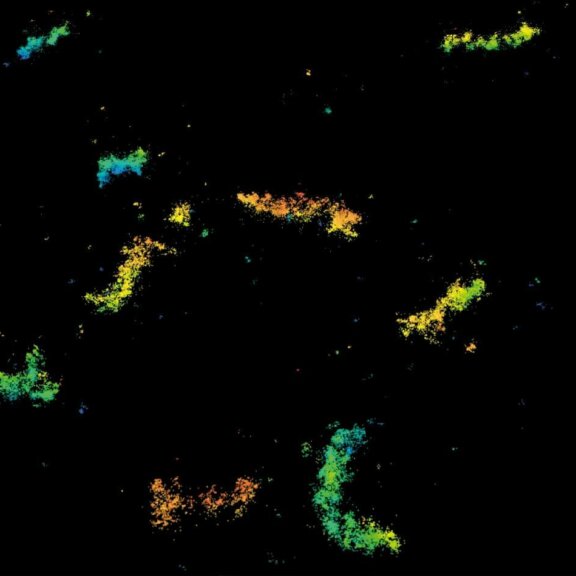
3D MINFLUX imaging of the peroxisomal membrane protein PMP70 labelled with abberior FLUX 647 in fixed mammalian cells using indirect immunofluorescence. 3D MINFLUX allows visualizing the shape of peroxisomes in all directions.
Description
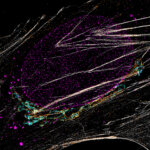
MINFLUX 3D imaging of Clathrin coated vesicles and Clathrin coated pits. Labeling was performed using clathrin light chain-SNAP together with SNAP-Alexa Fluor 647.
Description
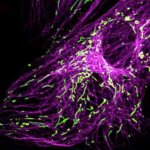
MINFLUX image of the mitochondrial import receptor Tom20 labeled with Alexa Fluor 647 in fixed mammalian cells using indirect immunolabeling. Please note that Tom20 is only localized at the mitochondrial surface.
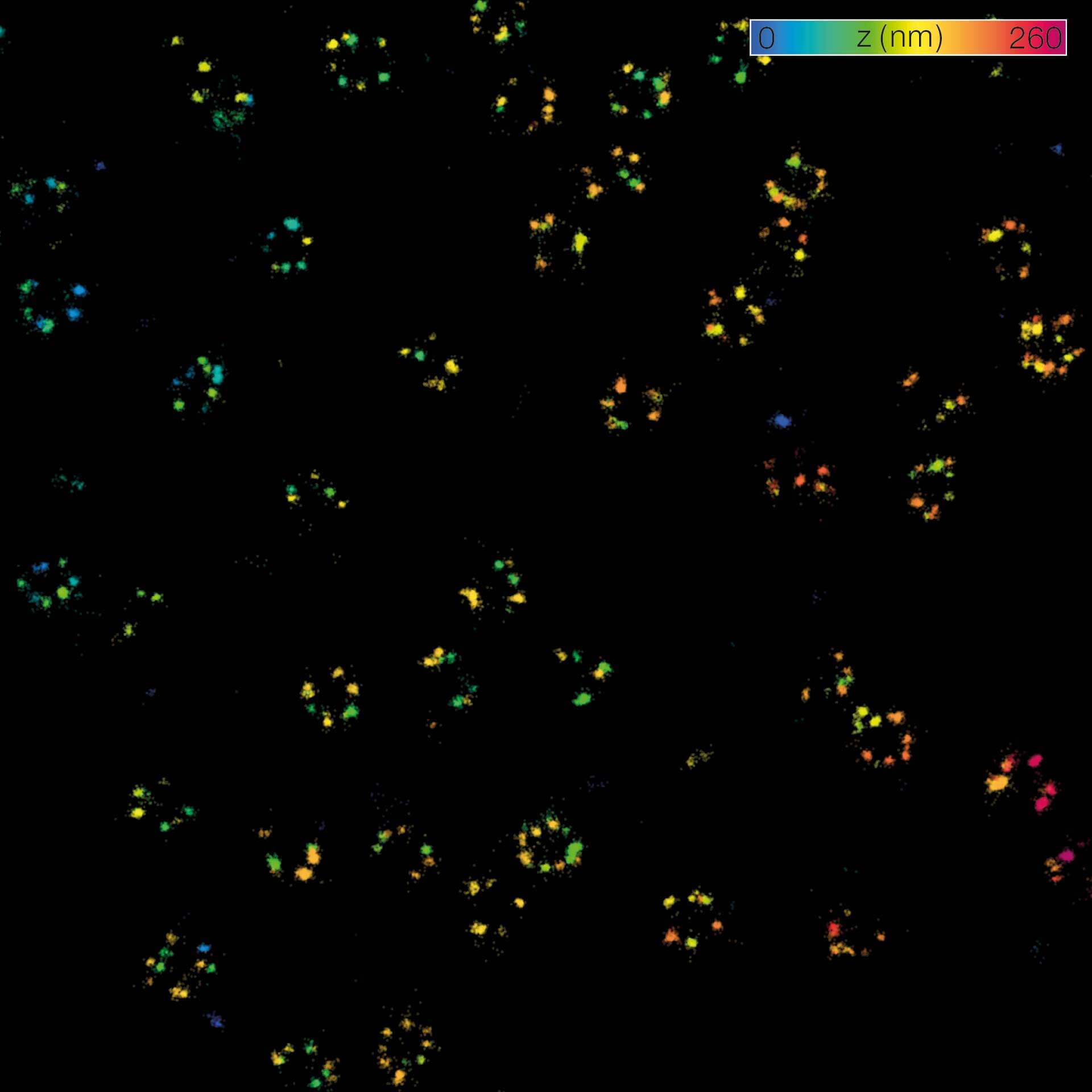
Description
MINFLUX 3D on nuclear pore complexes. Localization precision is better than 3 nm along all directions
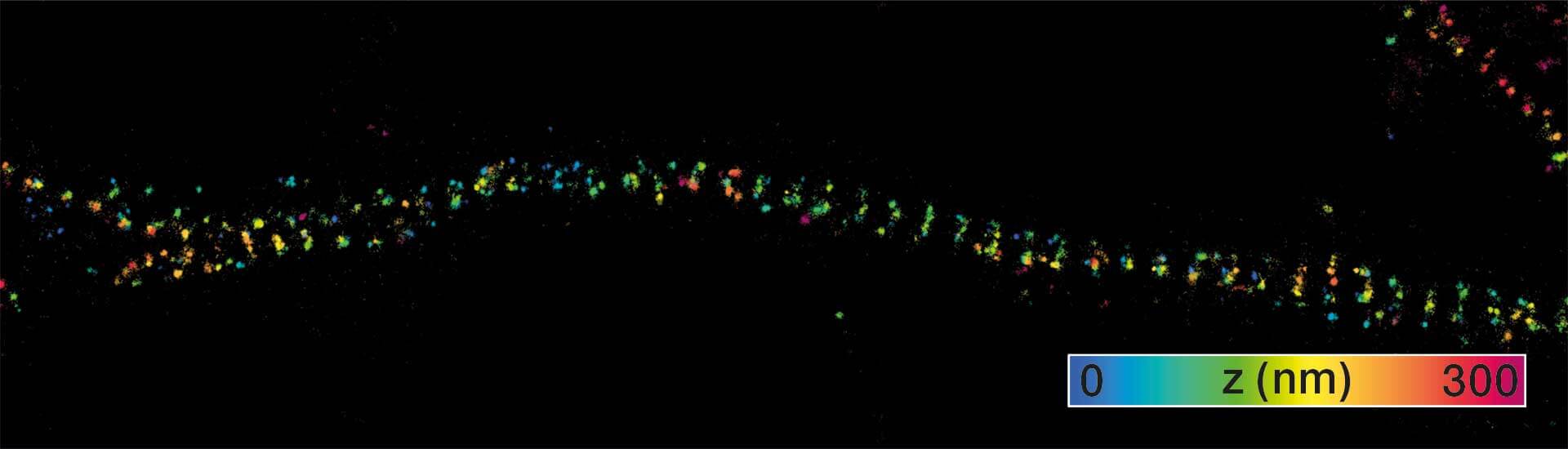
Description
3D MINFLUX imaging of βII spectrin in a primary hippocampal neuron labeled with Alexa Fluor 647 by indirect immunofluorescence
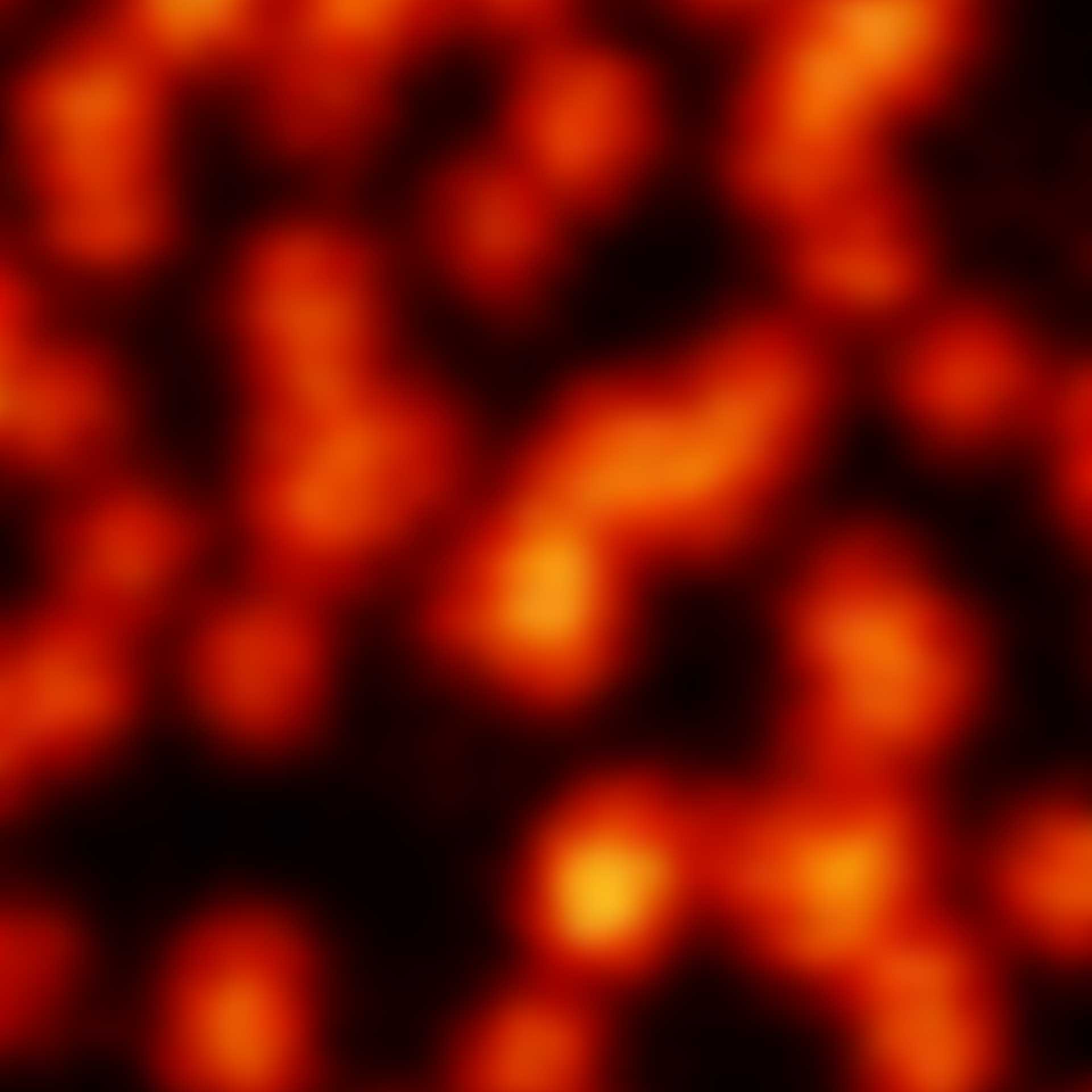

Description
2D MINFLUX nanoscopy of the nuclear pore complex subunits. NUP96-SNAP/SNAP-Alexa Fluor 647 lend themselves as benchmark structures to test superresolution light microscopes. In contrast to confocal microscopy, 2D MINFLUX allows to visualize the shape and arrangement of individual subunits of the nuclear pore complex. Here, we reach localization precisions of ~2 nm in raw localization data.

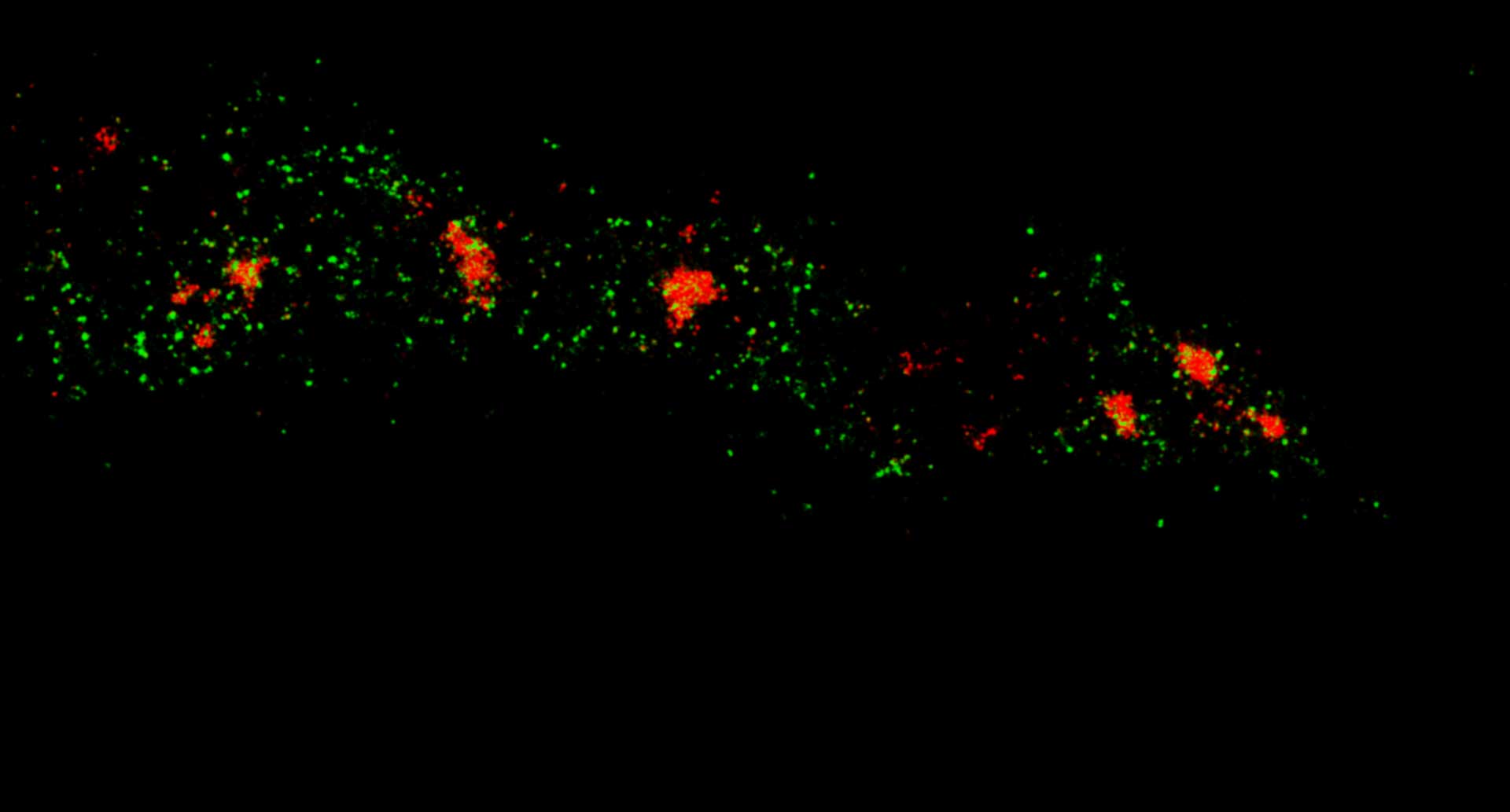
Description
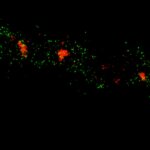
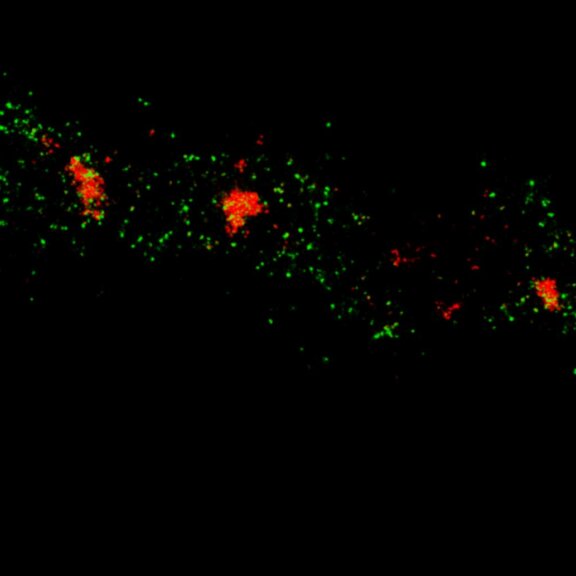
Two-color MINFLUX on mitochondria samples. The mitochondrial proteins TOM20 (green) and mtDNA (red) were labeled in mammalian cells with indirect immunofluorescence using secondary antibodies coupled to sCy5 and CF680. Two-color confocal (A) and MINFLUX (B) was performed using a ratiometric detection strategy. Please note that the labeling density of both structures is highly dissimilar. For TOM20 single proteins are labeled in the mitochondrial membrane, whereas numerous binding sites are decorated in the mtDNA. MINFLUX enables the visualization and separation of both structures.
Description
Autofluorescence in a plant stem cross section of Convallaria recorded with FACILITY. A large area, 10 by 10 tiles, each 125 µm by 125 µm, was imaged and stitched with image tiling in Fiji for ImageJ. This is combined with TIMEBOW lifetime imaging to show the differences in fluorescence lifetime due to the type of autofluorescent molecule and their nano-environment.
Modules:
Description
Live-cell sample of an Arabidopsis root tip suspended in water recorded with FACILITY. A subset of cells expresses a YFP construct. Lifetime imaging with TIMEBOW shows shifts in YFP fluorescence lifetime caused by the proteins nano-environment.
We thank Dr. Fábián Attila and Soós Vilmos (ATK, Brunszvik, HU) for providing this sample. Contact: soos.vilmos@atk.hu.
Modules:
Description
Four-color staining of fixed mammalian cells recorded with FACILITY showing nuclear pores (NUP98, STAR RED), Golgi membrane (GM130, STAR 635), Golgi medial rims (Giantin, STAR ORANGE) and actin (STAR 580 phalloidin). Shown left, just using spectral information, STAR RED and STAR 635 (magenta) cannot be separated, because of their similar excitation properties. The same goes for STAR ORANGE and STAR 580 (grey).
With the lifetime information collected by TIMEBOW, these dyes can be distinguished, turning an image with two recording channels into a four-color image. To achieve superresolution only one STED laser at 775 nm was used.
All utilized dyes are manufactured by abberior.
Modules:
Description
Autofluorescence in a plant stem cross section of Convallaria recorded with FACILITY. Image quality is improved by removing the background through MATRIX detection. This is combined with TIMEBOW lifetime imaging to show the differences in fluorescence lifetime due to the type of autofluorescent molecule and their nano-environment.
Modules:
Description
TIMEBOW STED vs MATRIX + TIMEBOW STED
Actin in microvilli of CaCo2 cells recorded with FACILITY. Fixed cells were stained with abberior STAR RED phalloidin. Imaging of the intense-background sample is optimized by MATRIX detection by directly subtracting unfocussed data. To boost resolution TIMEBOW is used, keeping STED laser power low. Lifetime information enables the selective exclusion of STED-affected emission at the rim of each measurement.
We thank Prof. Dorothee Günzel and Jörg Piontek (Charité, Berlin, DE) for providing this sample.
Modules:
Description
Confocal vs MATRIX + TIMEBOW STED
Actin in microvilli of CaCo2 cells recorded with FACILITY. Fixed cells were stained with abberior STAR RED phalloidin. Imaging of the intense-background sample is optimized by MATRIX detection by directly subtracting unfocussed data. To boost resolution TIMEBOW is used, keeping STED laser power low. Lifetime information enables the selective exclusion of STED-affected emission at the rim of each measurement.
We thank Prof. Dorothee Günzel and Jörg Piontek (Charité, Berlin, DE) for providing this sample.
Modules:
Description
MINFLUX 3D imaging of βII spectrin in a primary hippocampal neuron labeled with Alexa Fluor 647 by indirect immunofluorescence. The axial coordinate is color-coded. Please note the periodic arrangement of spectrin along the axon.
Description
MINFLUX 3D nanoscopy of nuclear pore complex subunits. 3D MINFLUX facilitates 3D imaging at molecular scales. In the axial direction localization, we reach precisions of approx. 2.5 nm in raw localization data. Labeling of NUP96-SNAP was performed using SNAP-Alexa Fluor 647.
Description
3D MINFLUX imaging of the peroxisomal membrane protein PMP70 labelled with abberior FLUX 647 in fixed mammalian cells using indirect immunofluorescence. 3D MINFLUX allows visualizing the shape of peroxisomes in all directions.
Description
MINFLUX 3D imaging of Clathrin coated vesicles and Clathrin coated pits. Labeling was performed using clathrin light chain-SNAP together with SNAP-Alexa Fluor 647.
Description
MINFLUX image of the mitochondrial import receptor Tom20 labeled with Alexa Fluor 647 in fixed mammalian cells using indirect immunolabeling. Please note that Tom20 is only localized at the mitochondrial surface.
Description
MINFLUX 3D on nuclear pore complexes. Localization precision is better than 3 nm along all directions
Description
3D MINFLUX imaging of βII spectrin in a primary hippocampal neuron labeled with Alexa Fluor 647 by indirect immunofluorescence
Description
3D MINFLUX allowes to visualize the full shape of peroxisomes.
Description
2D MINFLUX nanoscopy of the nuclear pore complex subunits. NUP96-SNAP/SNAP-Alexa Fluor 647 lend themselves as benchmark structures to test superresolution light microscopes. In contrast to confocal microscopy, 2D MINFLUX allows to visualize the shape and arrangement of individual subunits of the nuclear pore complex. Here, we reach localization precisions of ~2 nm in raw localization data.
Description
Two-color MINFLUX on mitochondria samples. The mitochondrial proteins TOM20 (green) and mtDNA (red) were labeled in mammalian cells with indirect immunofluorescence using secondary antibodies coupled to sCy5 and CF680. Two-color confocal (A) and MINFLUX (B) was performed using a ratiometric detection strategy. Please note that the labeling density of both structures is highly dissimilar. For TOM20 single proteins are labeled in the mitochondrial membrane, whereas numerous binding sites are decorated in the mtDNA. MINFLUX enables the visualization and separation of both structures.