Sample gallery
Fluorescence imaging, whether at confocal, STED or MINFLUX resolution, guarantees unique insights into the function and structure of life at the molecular level. Besides the scientific information content, some sample portraits provide simply beautiful images. Enjoy browsing our sample gallery.
the fine art of science

Description
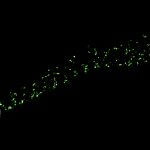
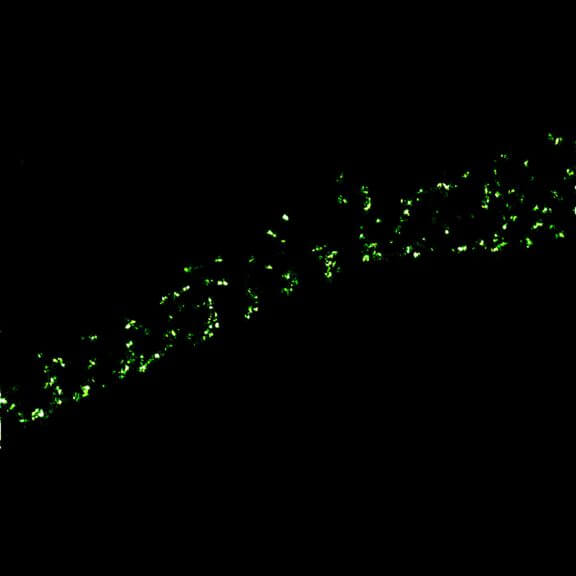
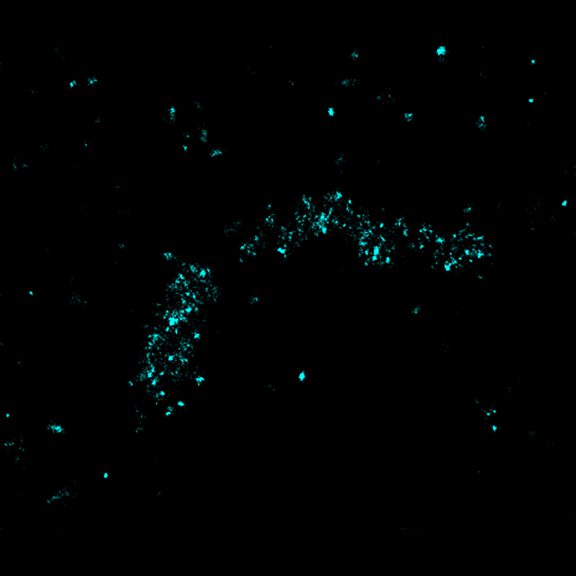
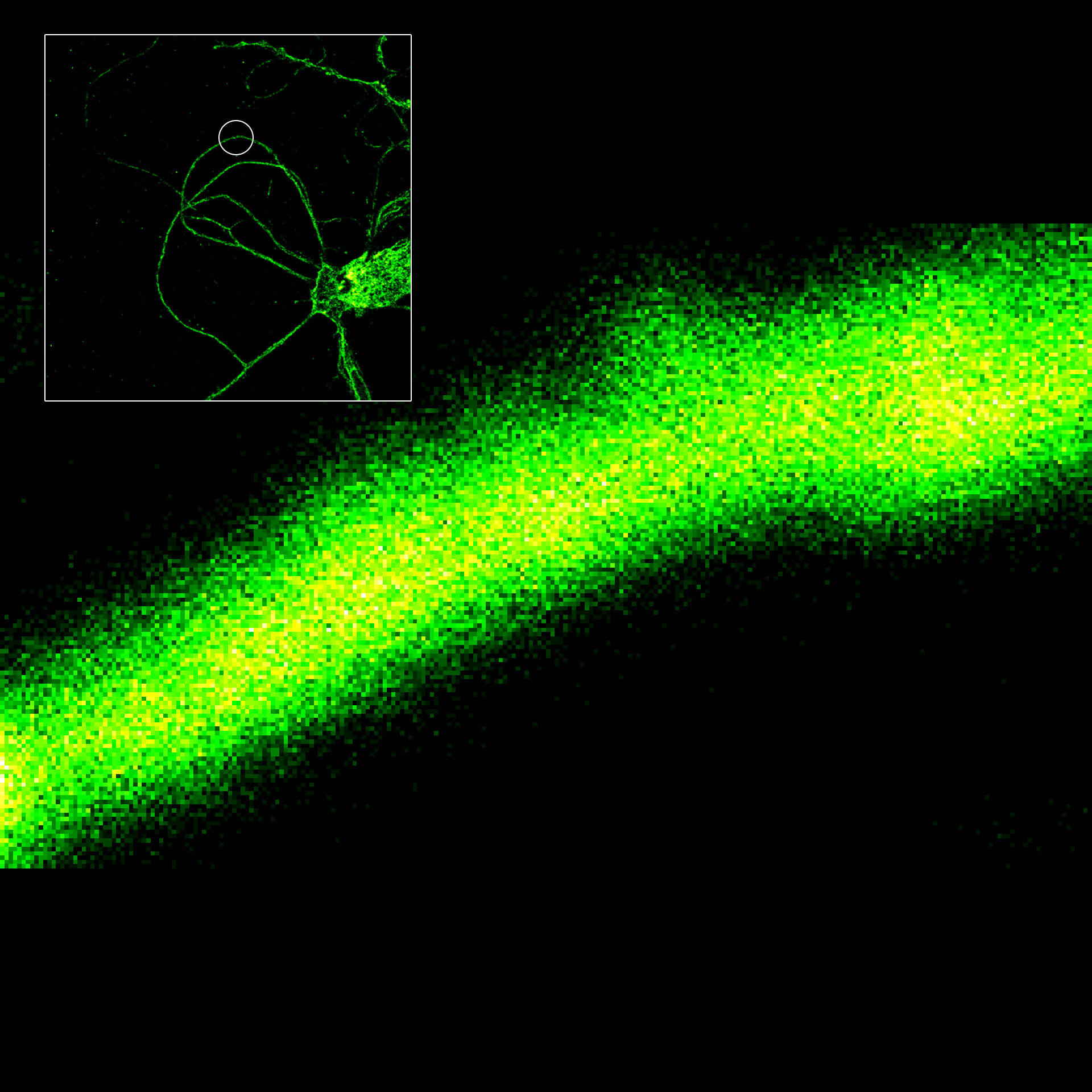
MINFLUX imaging of βII spectrin in a primary hippocampal neuron labeled with abberior FLUX 680 by indirect immunofluorescence. Please note the periodic arrangement of spectrin along the axon.
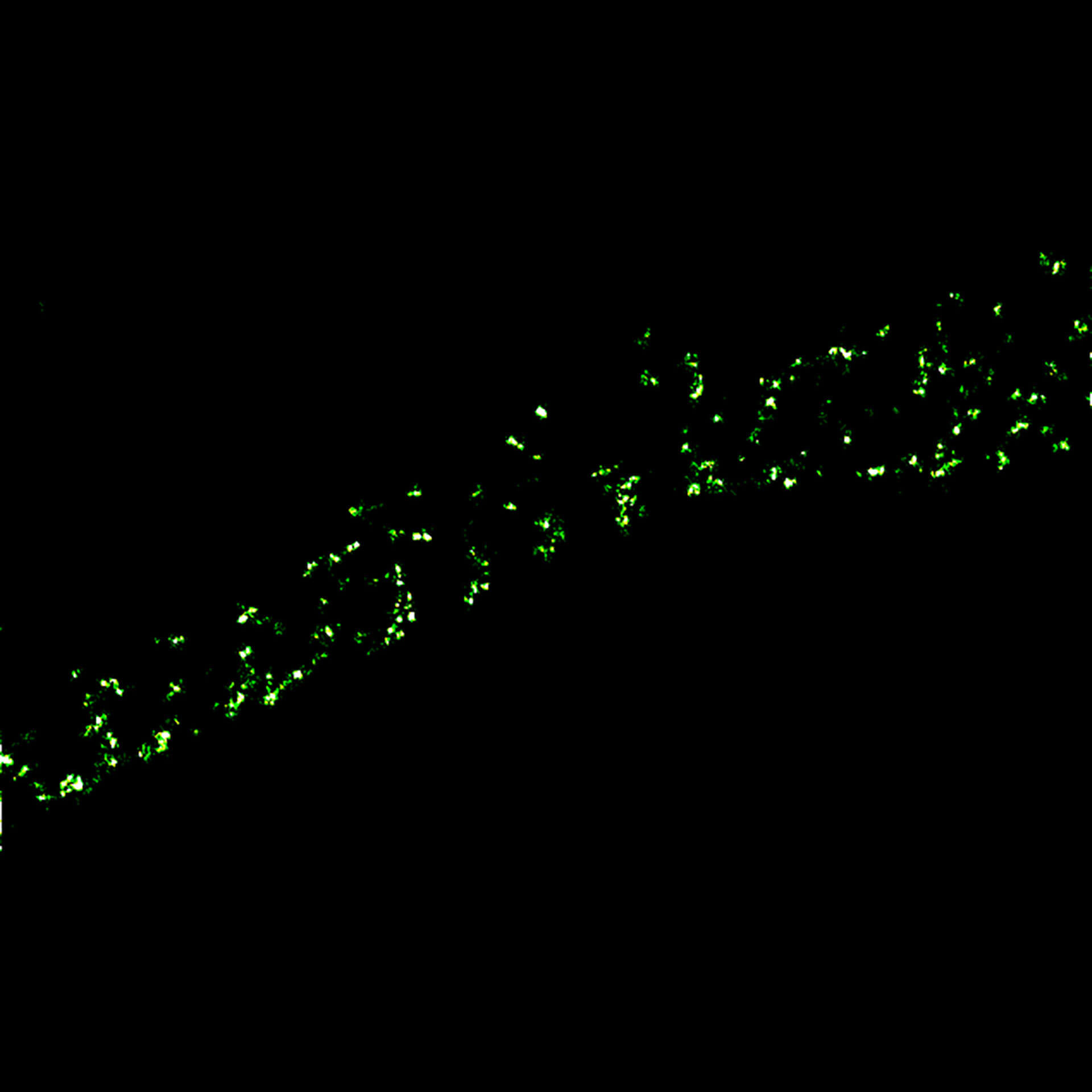
Description
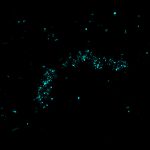
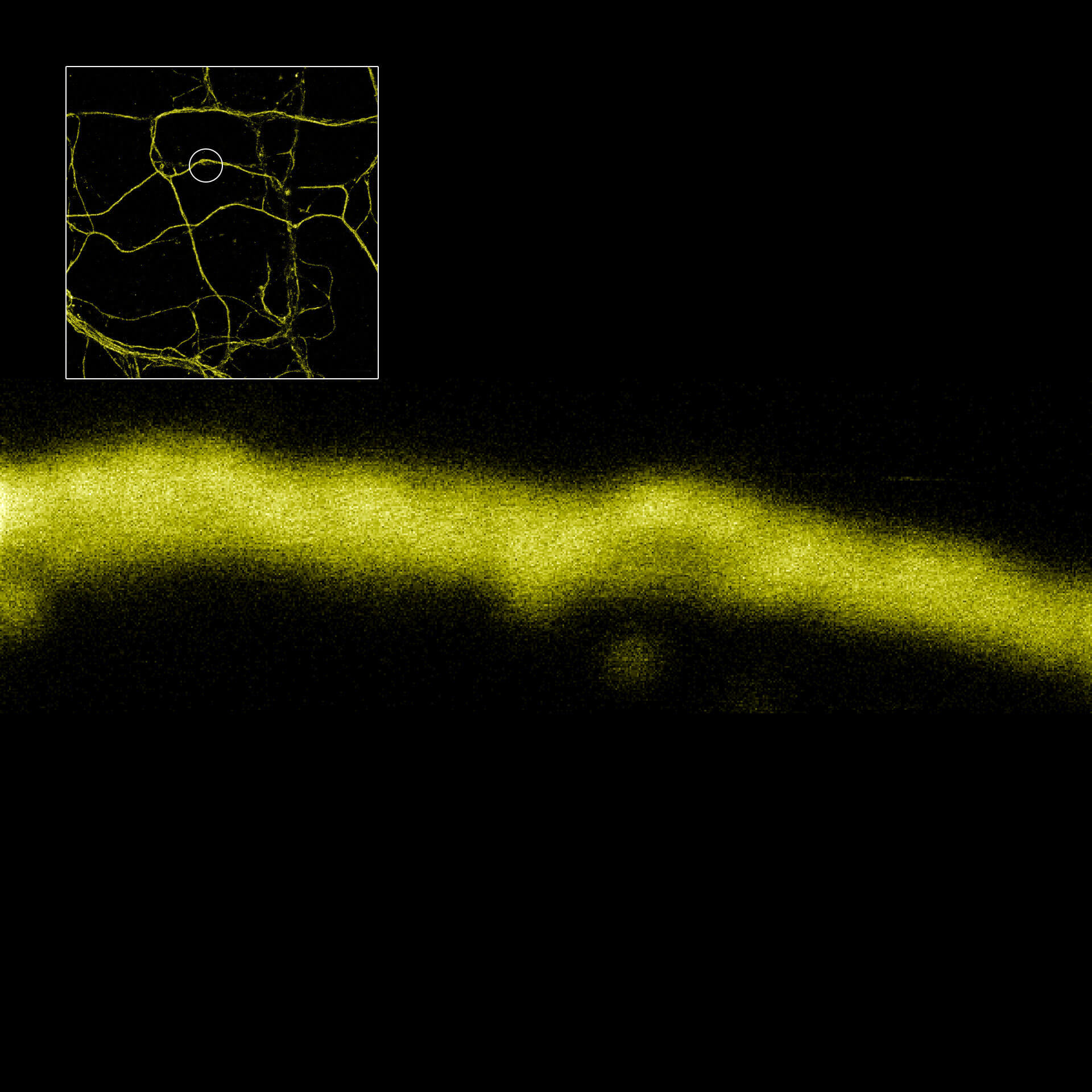
MINFLUX image of axonal βII spectrin labeled with abberior FLUX 660 in primary hippocampal neurons. Note the periodic arrangement of spectrin along the axon, and the absence of any details in the confocal counterpart image.
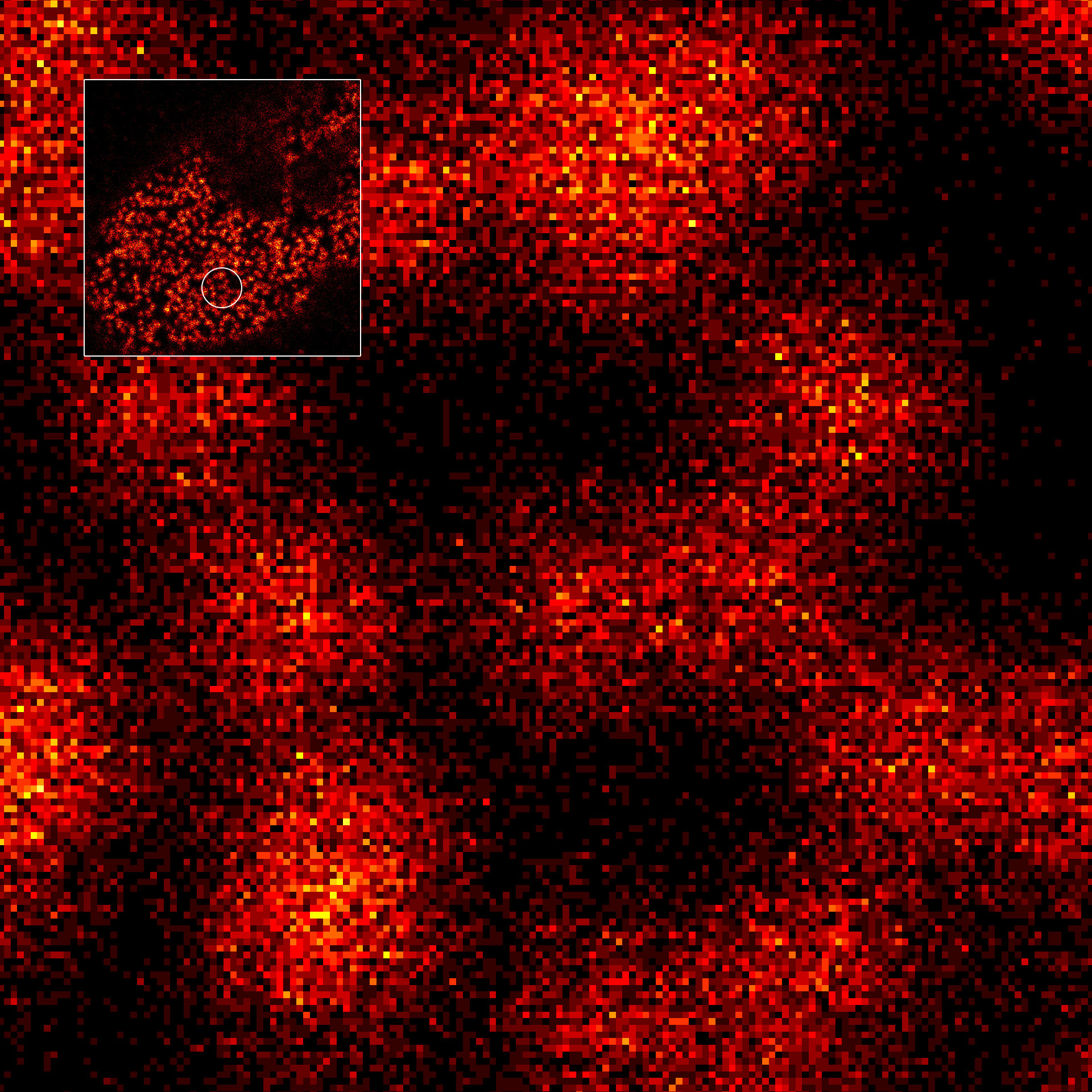

Description
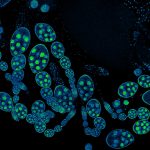
2D MINFLUX nanoscopy of the nuclear pore complex subunits. Fixed mammalian cells expressing SNAP-tag® NUP96 were labeled with abberior FLUX 647 SNAP. In contrast to confocal microscopy, 2D MINFLUX allows to visualize the shape and arrangement of individual subunits of the nuclear pore complex.
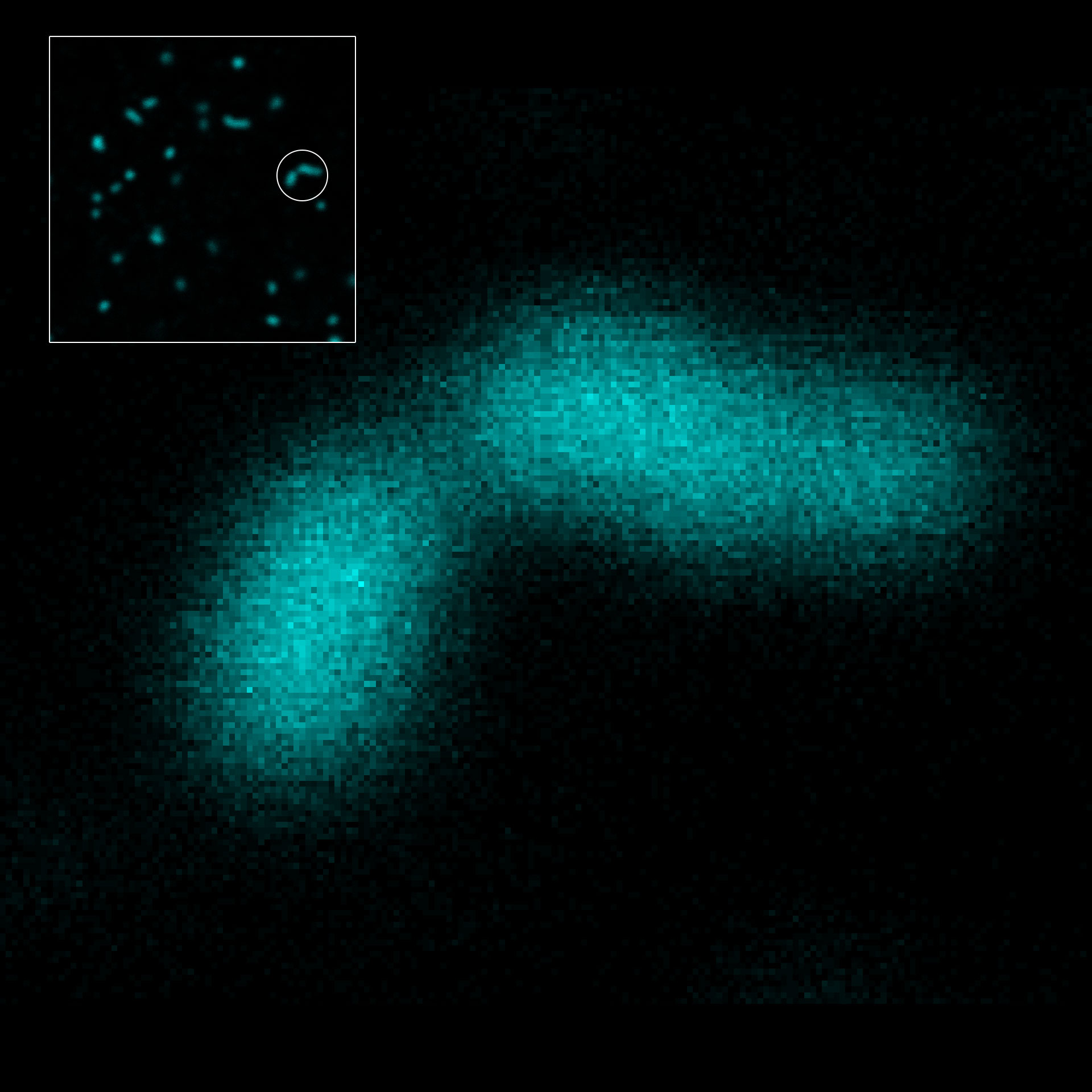
Description
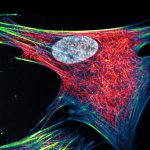
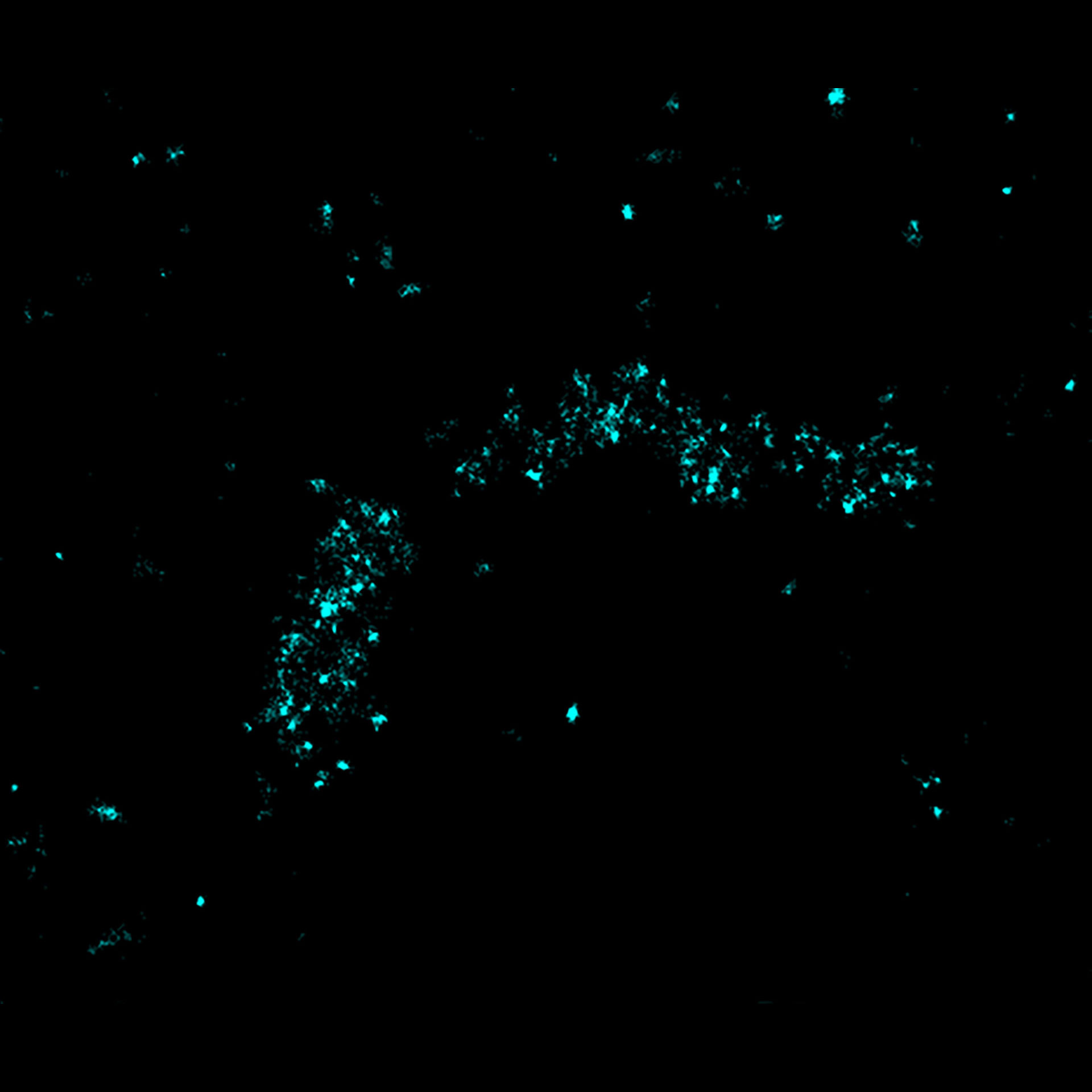
2D MINFLUX imaging of the peroxisomal membrane protein PMP70 labeled with abberior FLUX 640 in fixed mammalian cells using indirect immunofluorescence.
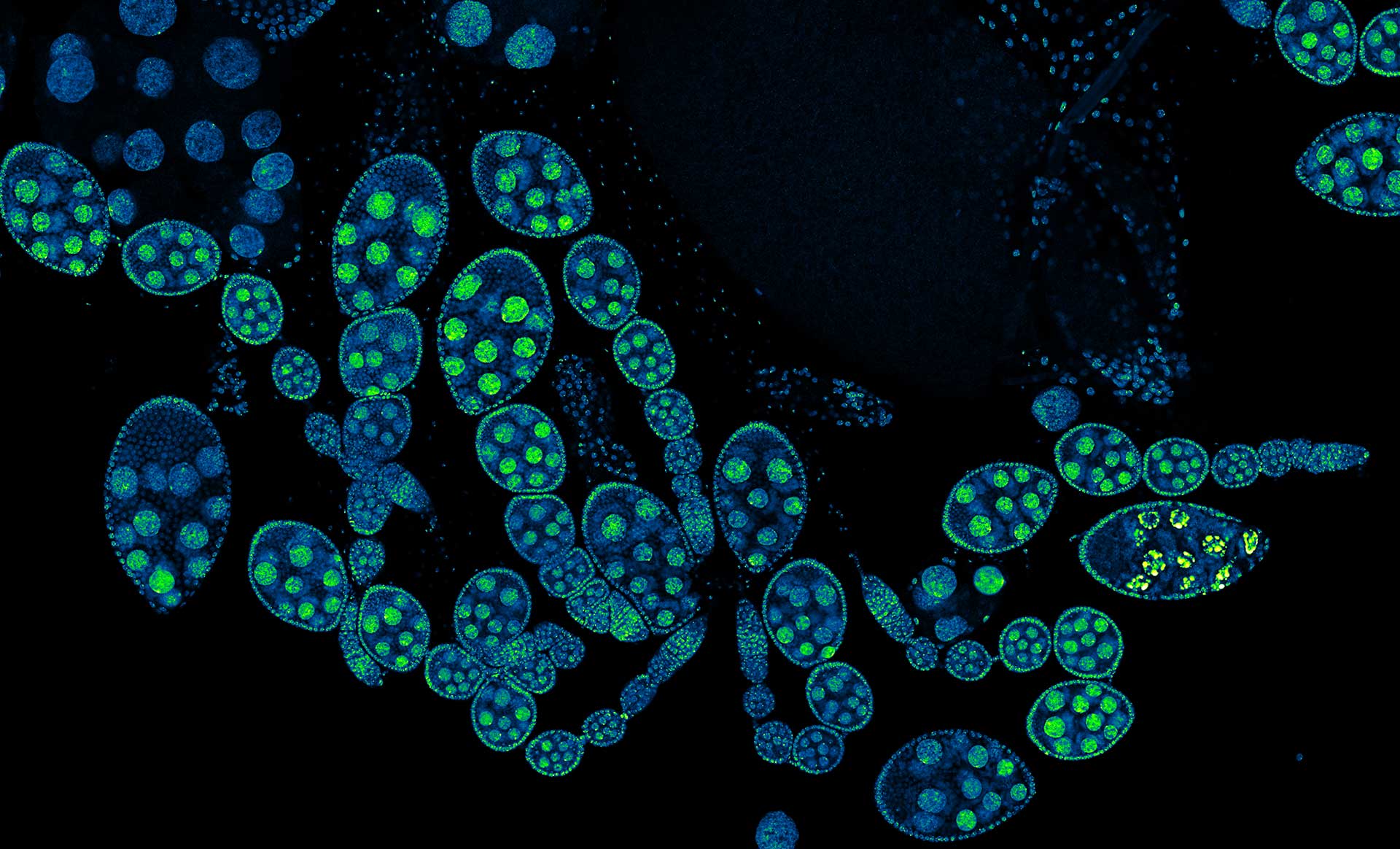
Description
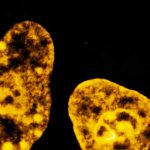
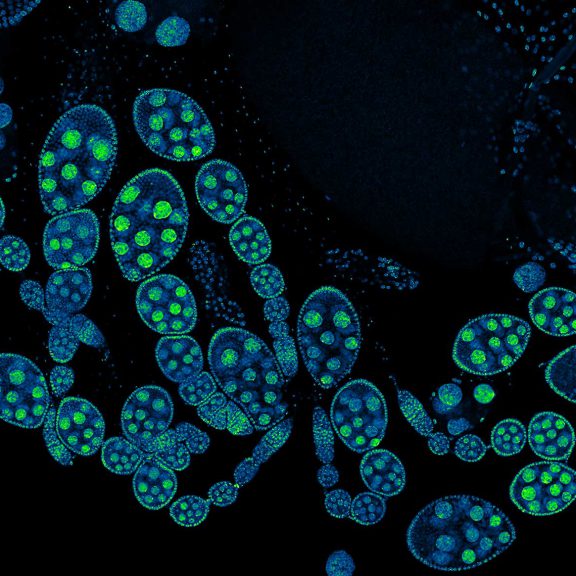
Drosophila ovariole stained with abberior LIVE 560 DNA showing nuclei in different cell types of the egg chamber. Ovaries were dissected from adult female fruit flies and were fixed prior to staining.
Image was acquired with the STEDYCON tiling feature and assembled with the SVI Huygens Stitcher.

Description
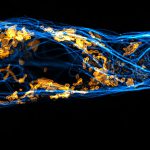
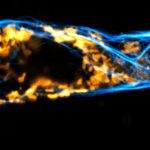
Drosophila spermatid tails were stained with abberior LIVE 610 tubulin. Testis were dissected from adult male fruit flies. Live cell imaging experiment was performed on a INFINITY microscope.
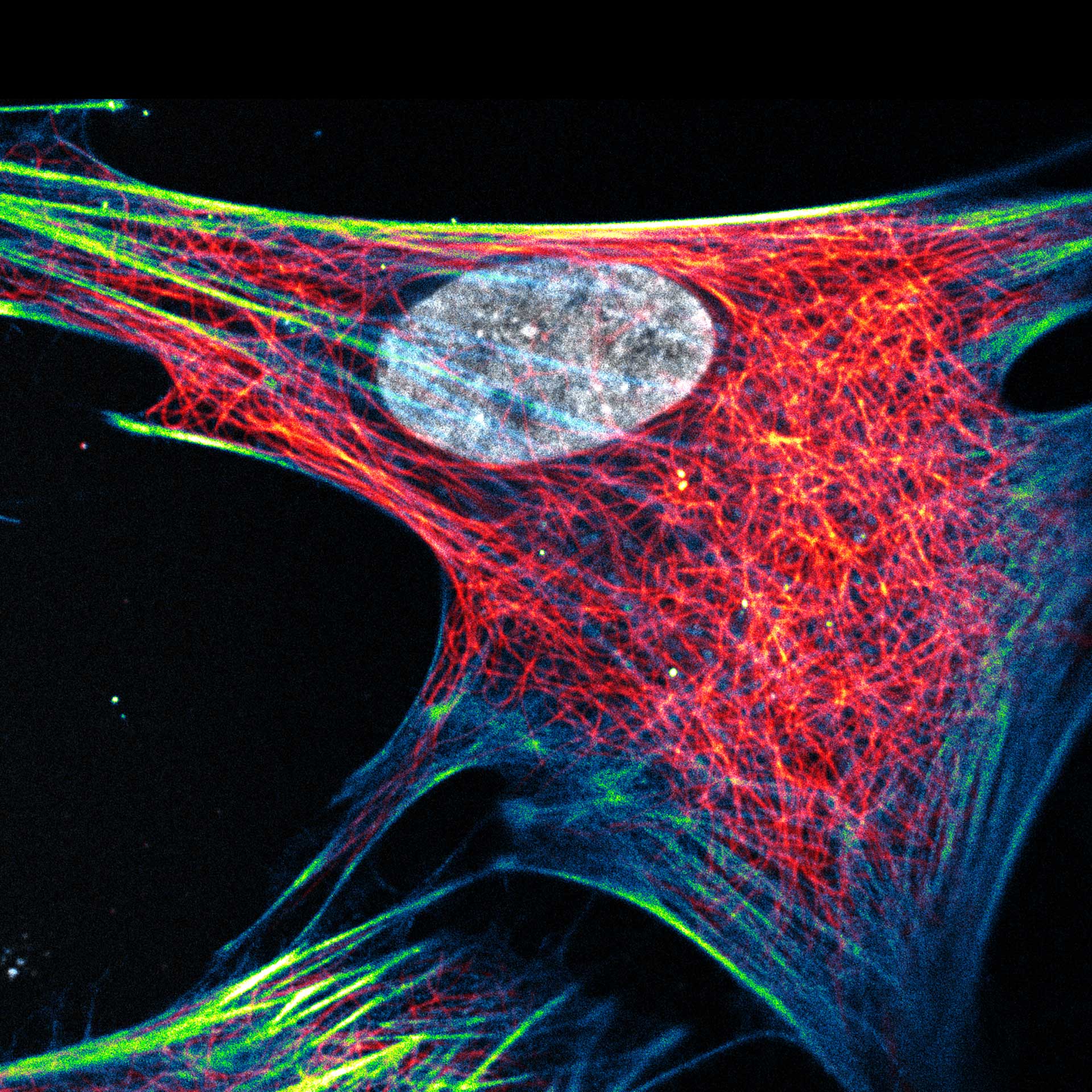
Description
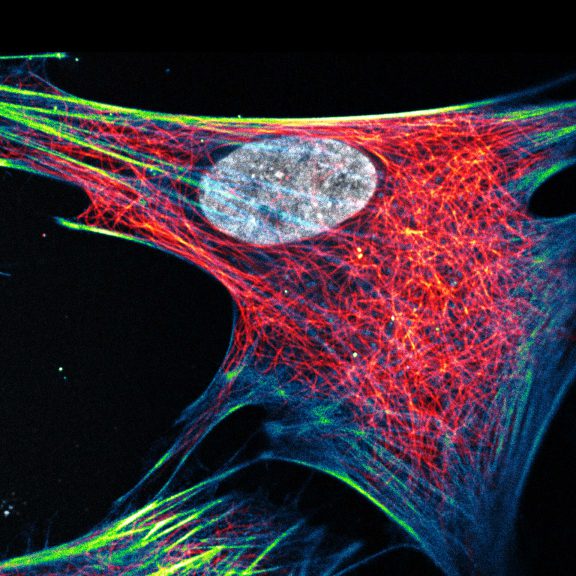
Composite of three color live-cell image of an adherent mammalian cell. This living cell was directly labelled with abberior LIVE 510 actin (blue/green), LIVE 560 DNA (gray) and LIVE 610 tubulin. This image was acquired with a STEDYCON microscope.
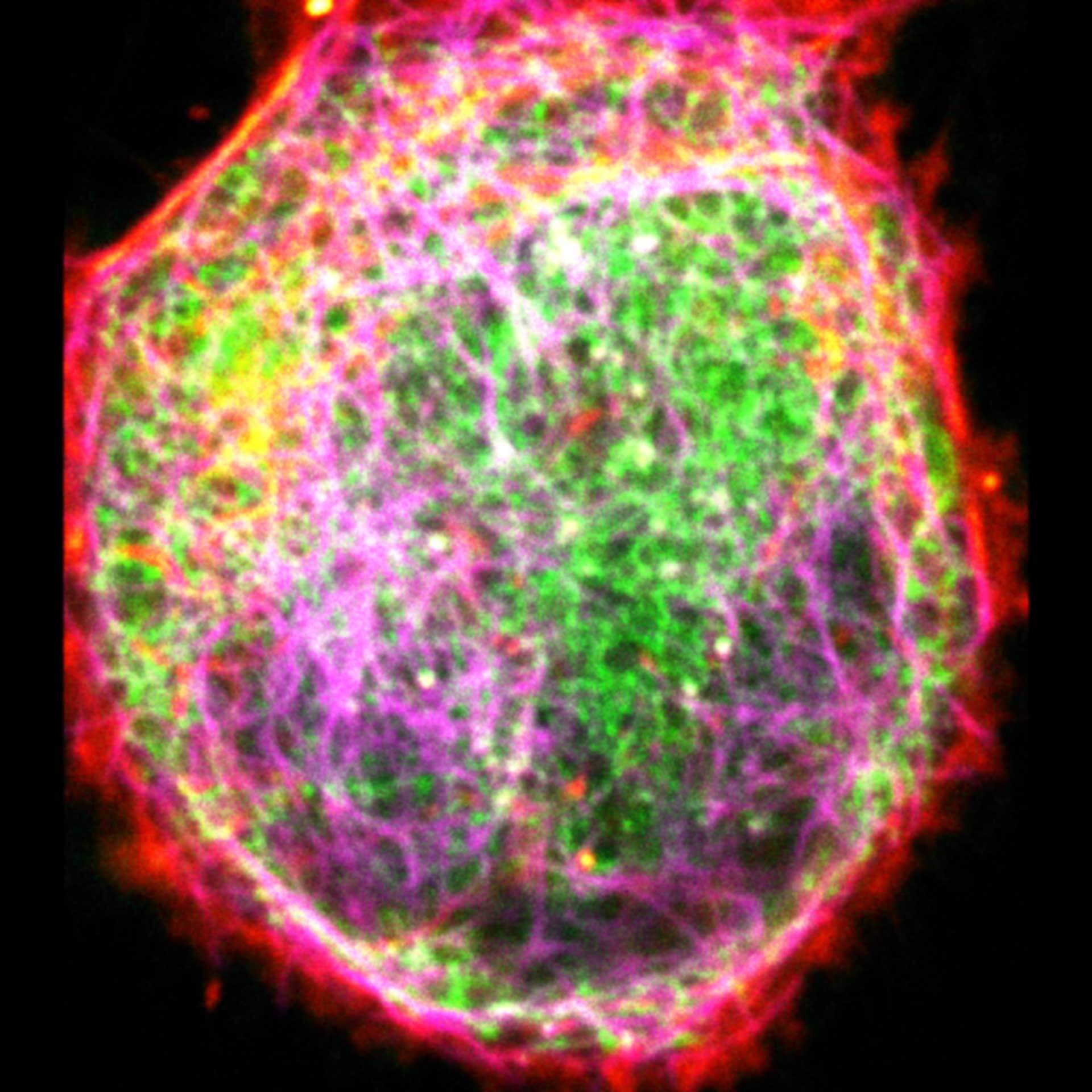
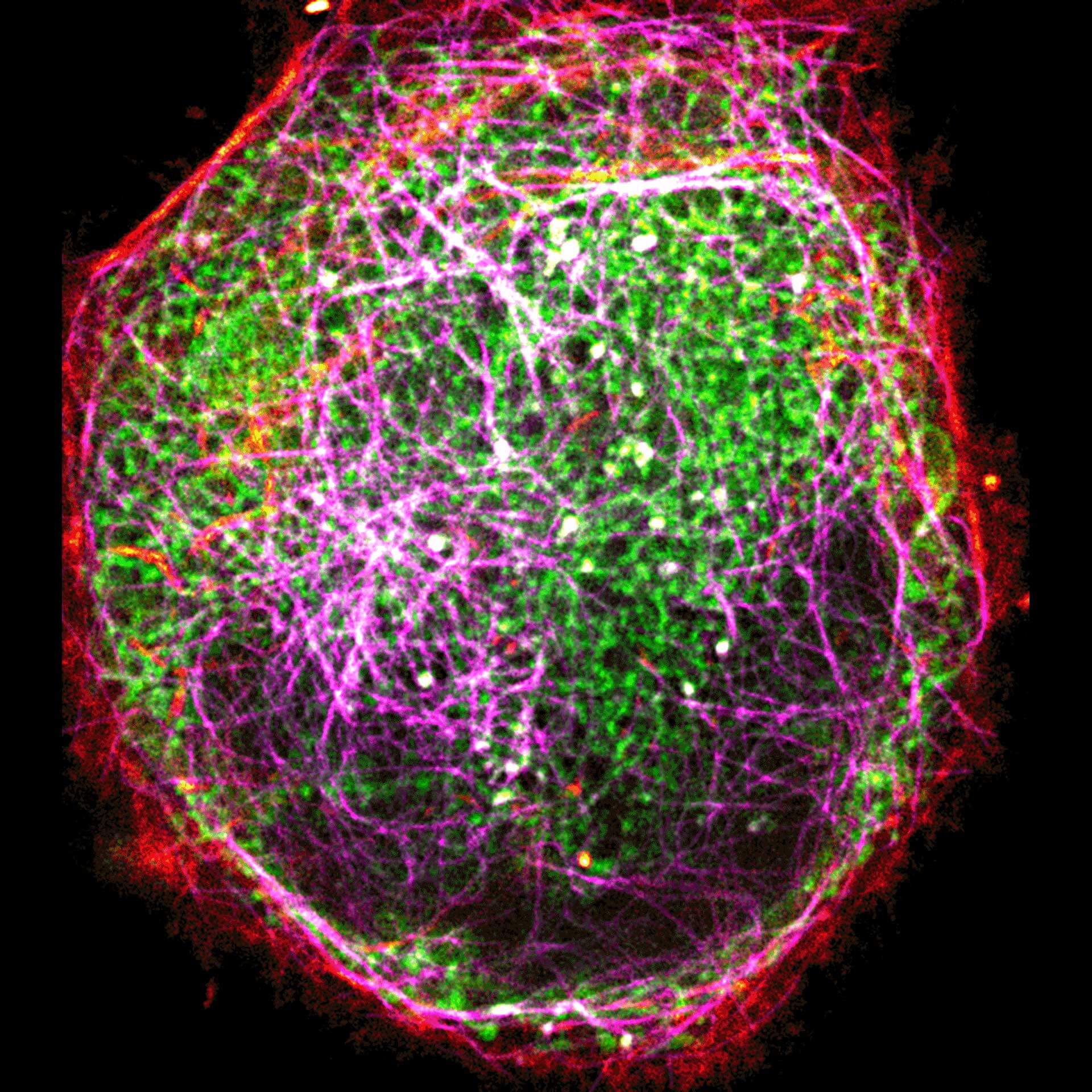
Description
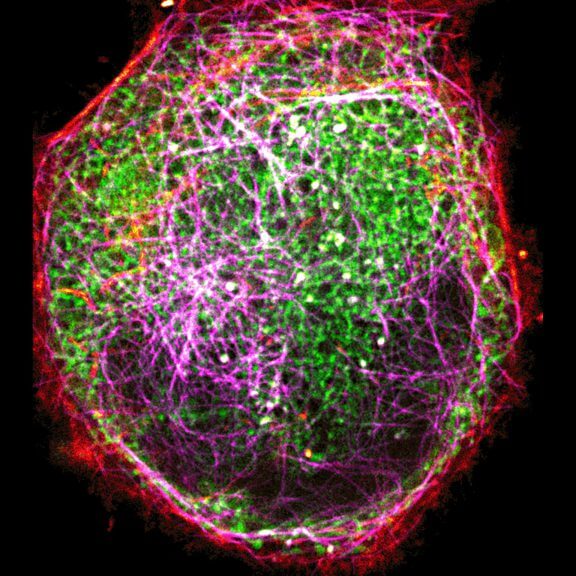
Three color live-cell STED at 775 nm: living cell labelled with abberior LIVE 460L (ER, green), LIVE 560 tubulin (magenta) and LIVE 610 actin (red).
Description
Confocal and STED time lapse of a living cell expressing SNAP-tag® fusion protein fusion protein in the endoplasmic reticulum lumen with SNAP and C-terminal tetrapeptide KDEL. SNAP-KDEL was labelled with abberior LIVE 610 SNAP ligand (benzylguanine).
The SNAP-KDEL plasmid was a gift from Francesca Bottanelli, FU Berlin.
Description
Live cell time lapse of abberior LIVE 590 DNA probe in cultured mammalian cells.
The use of very low concentrations of our abberior LIVE dyes reduces toxicity and allows long-term imaging of dynamic processes.
Image was acquired with the FACILITY microscope.
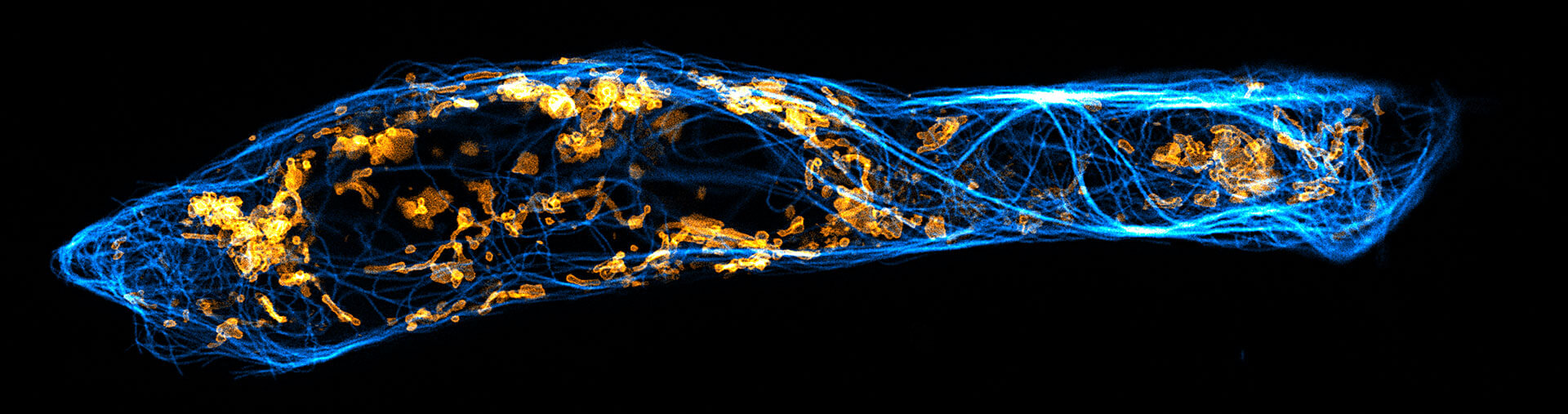
Description
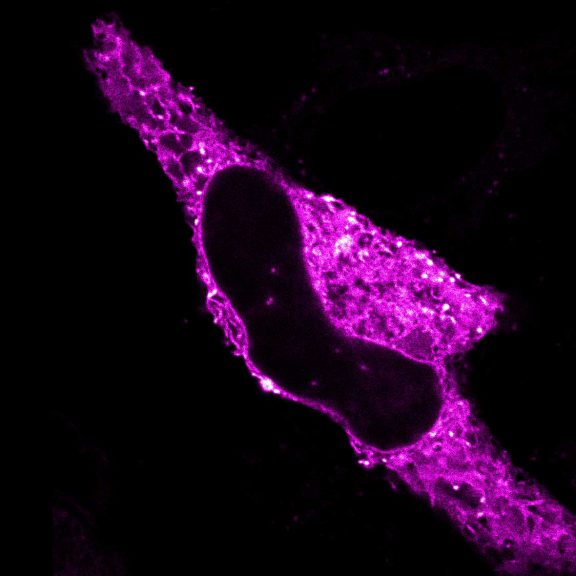
Two color live-cell confocal and STED image of a mammalian cell expressing a SNAP-tag® OMP25 fusion protein decoration the outer membrane of mitochondria. OMP25 is visualized by our new abberior LIVE 610 SNAP ligand (orange). Tubulin filaments are highlighted with abberior LIVE 550 tubulin (cyan).
The SNAP-OMP25 plasmid was a gift from Francesca Bottanelli, FU Berlin.
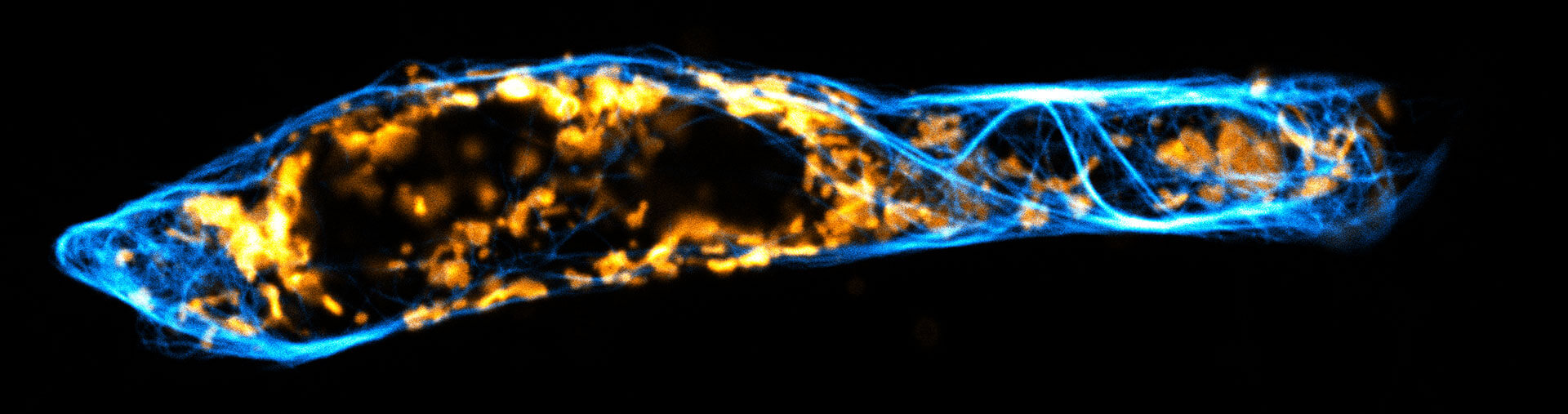

Description
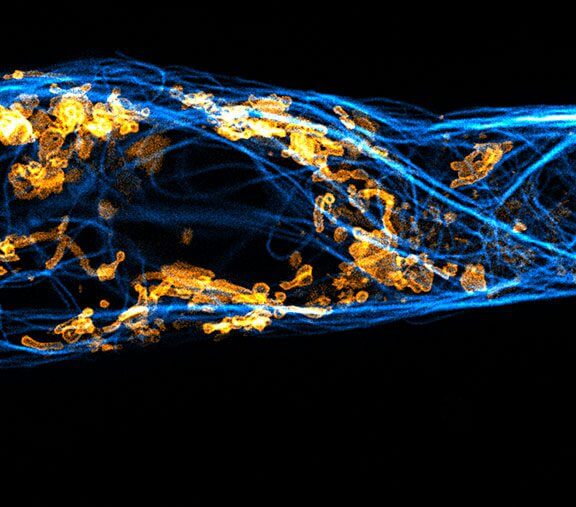
Two color live-cell confocal and STED image of a mammalian cell expressing a SNAP-tag® OMP25 fusion protein decoration the outer membrane of mitochondria. OMP25 is visualized by our new abberior LIVE 610 SNAP ligand (orange). Tubulin filaments are highlighted with abberior LIVE 550 tubulin (cyan).
The SNAP-OMP25 plasmid was a gift from Francesca Bottanelli, FU Berlin.
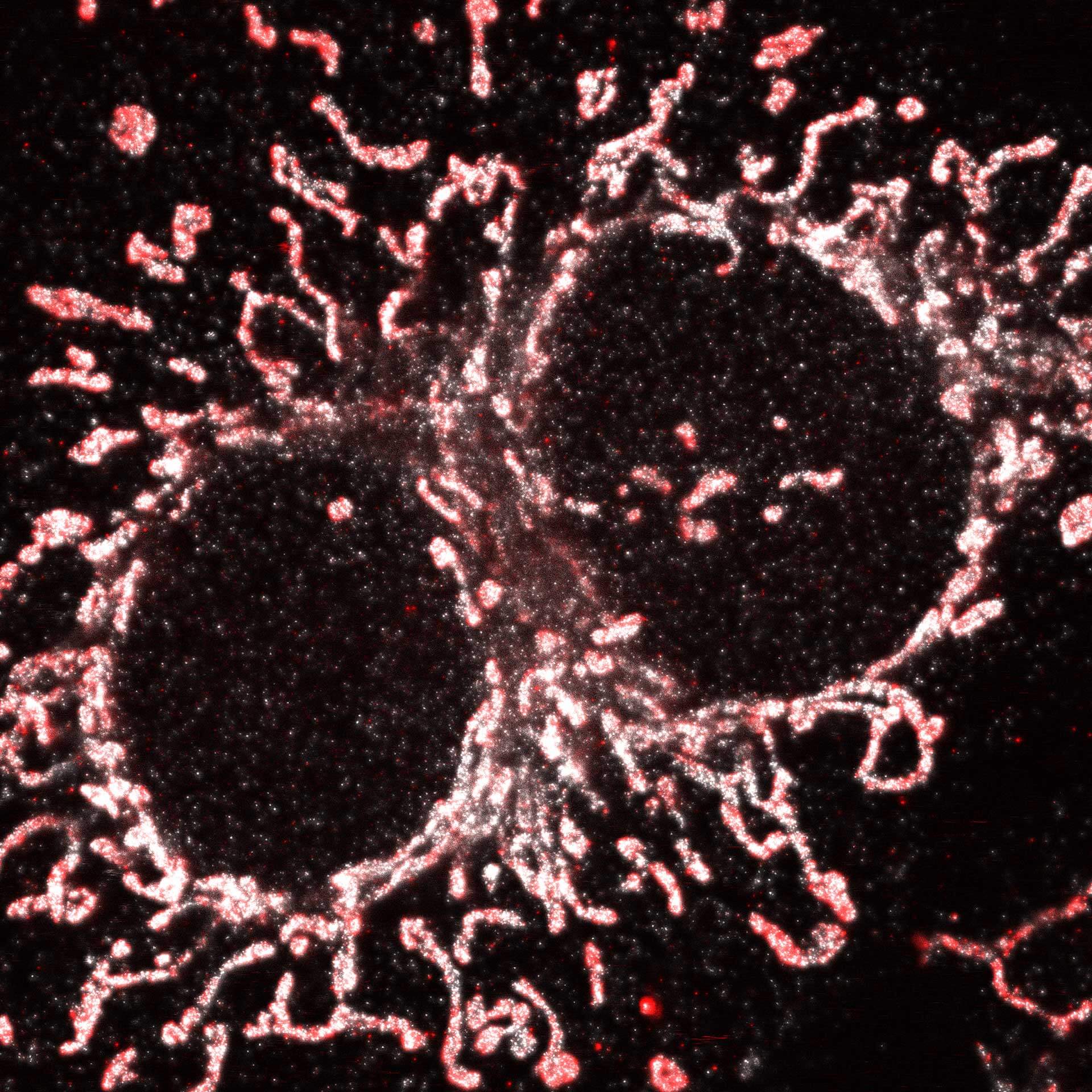
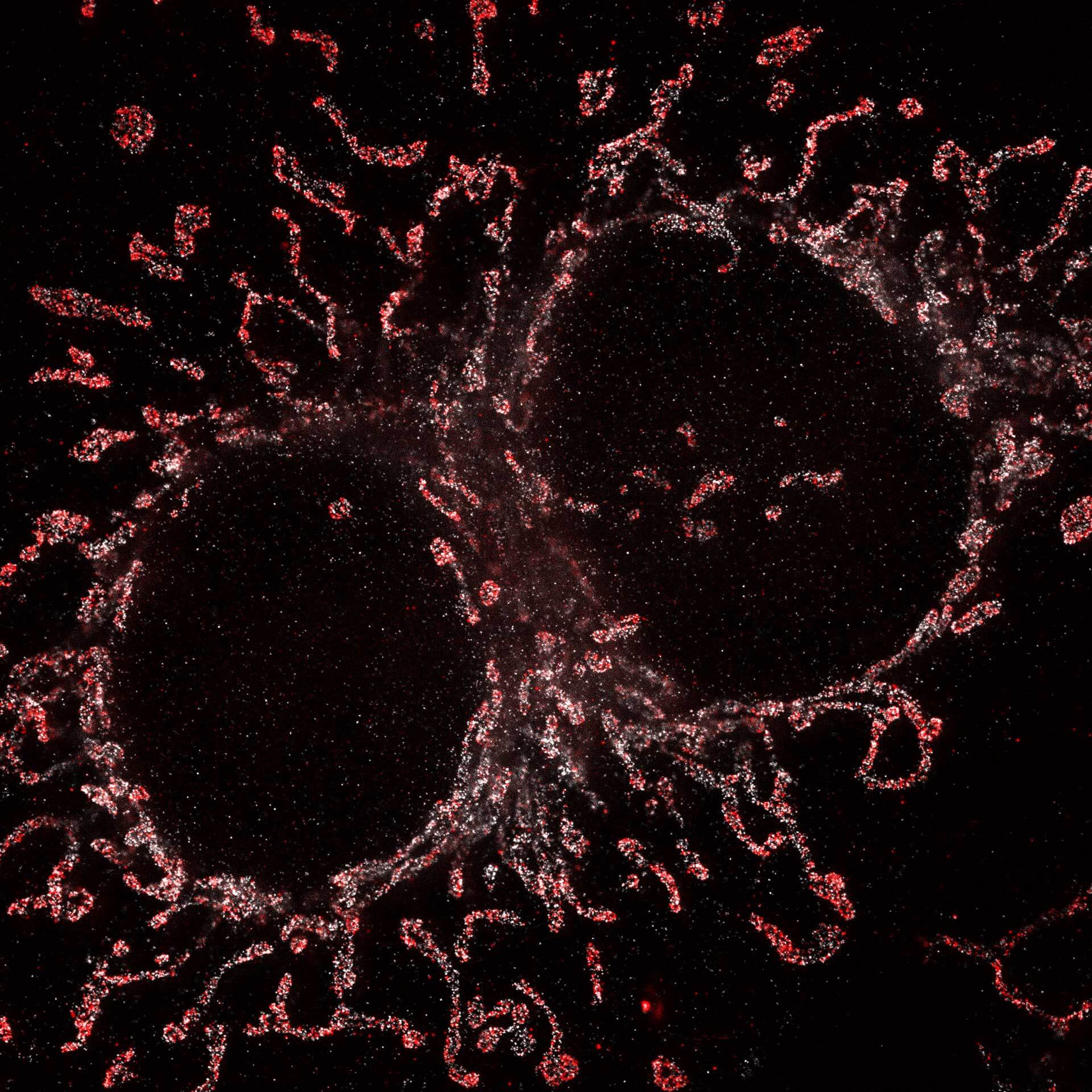
Description
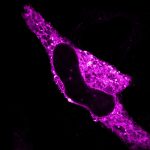
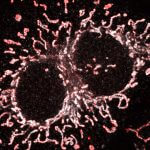
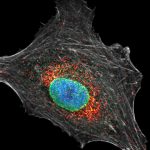
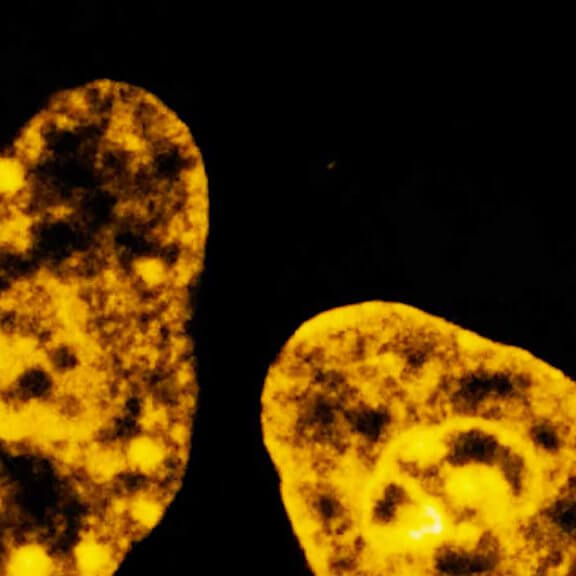
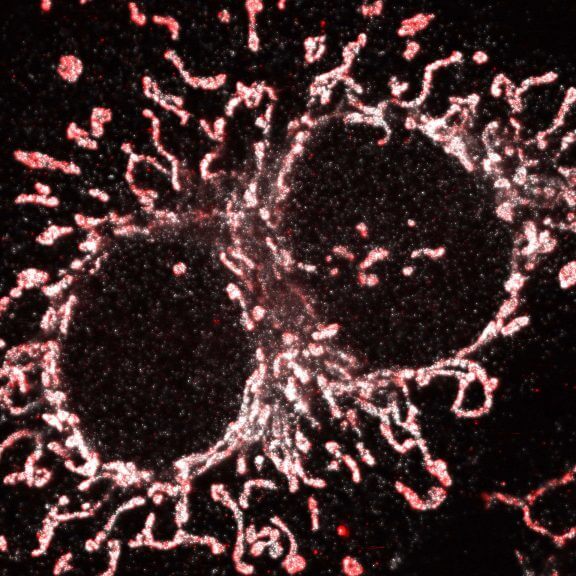
Cultured mammalian cell immunostained for an inner and outer mitochondrial membrane marker. Outer membrane is highlighted with abberior STAR RED (red) and the inner membrane with abberior STAR ORANGE (gray).
STED and confocal images were acquired with abberior's FACILITY microscope.
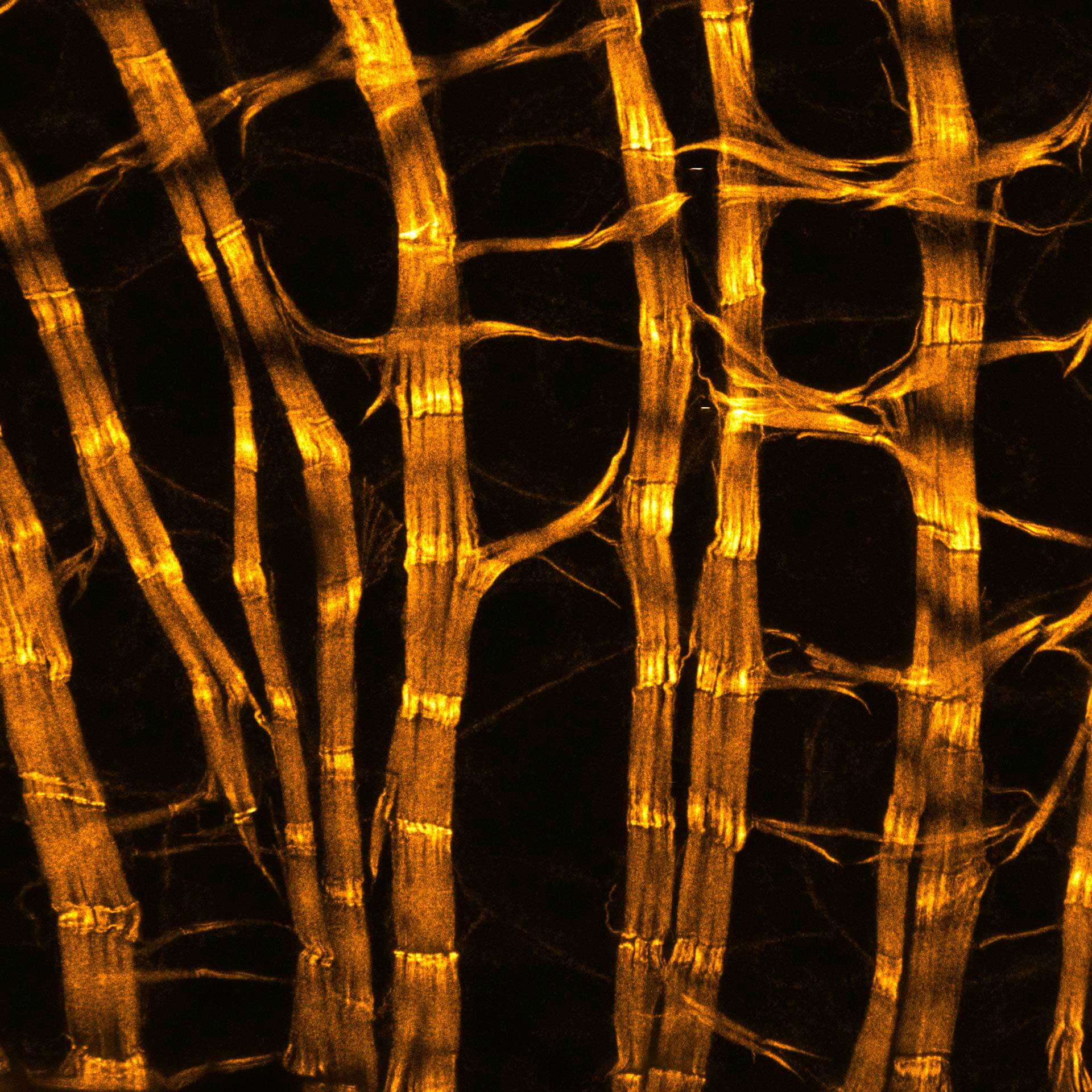
Description
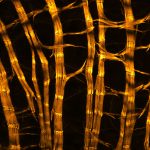
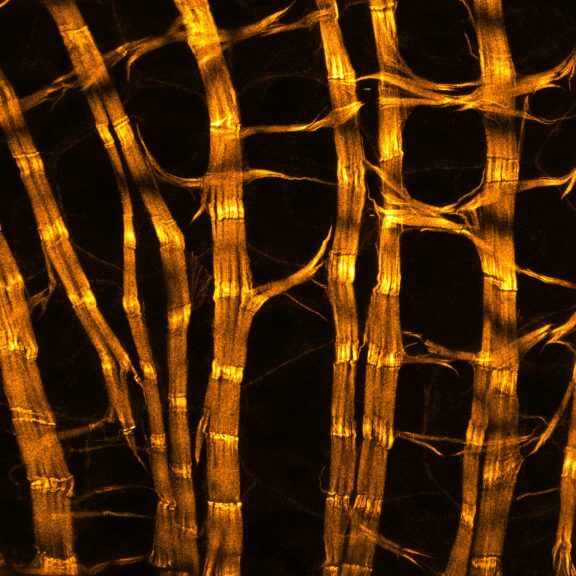
Drosophila male accessory gland stained for F-actin using abberior STAR 580 phalloidin.
Sample was prepared in cooperation with Dr. H. R. Shcherbata at MPl for Biophysical Chemistry, Göttingen, Germany.
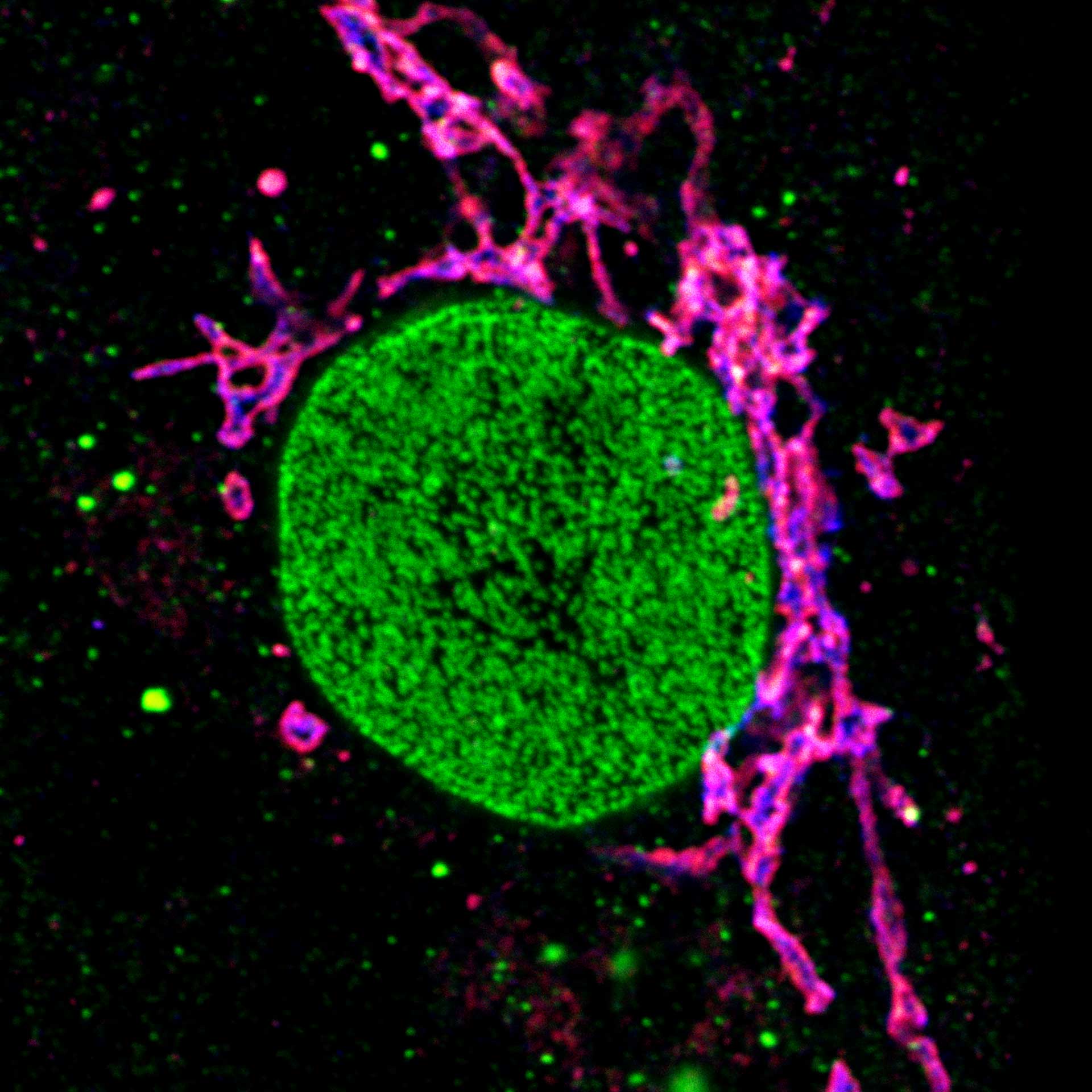
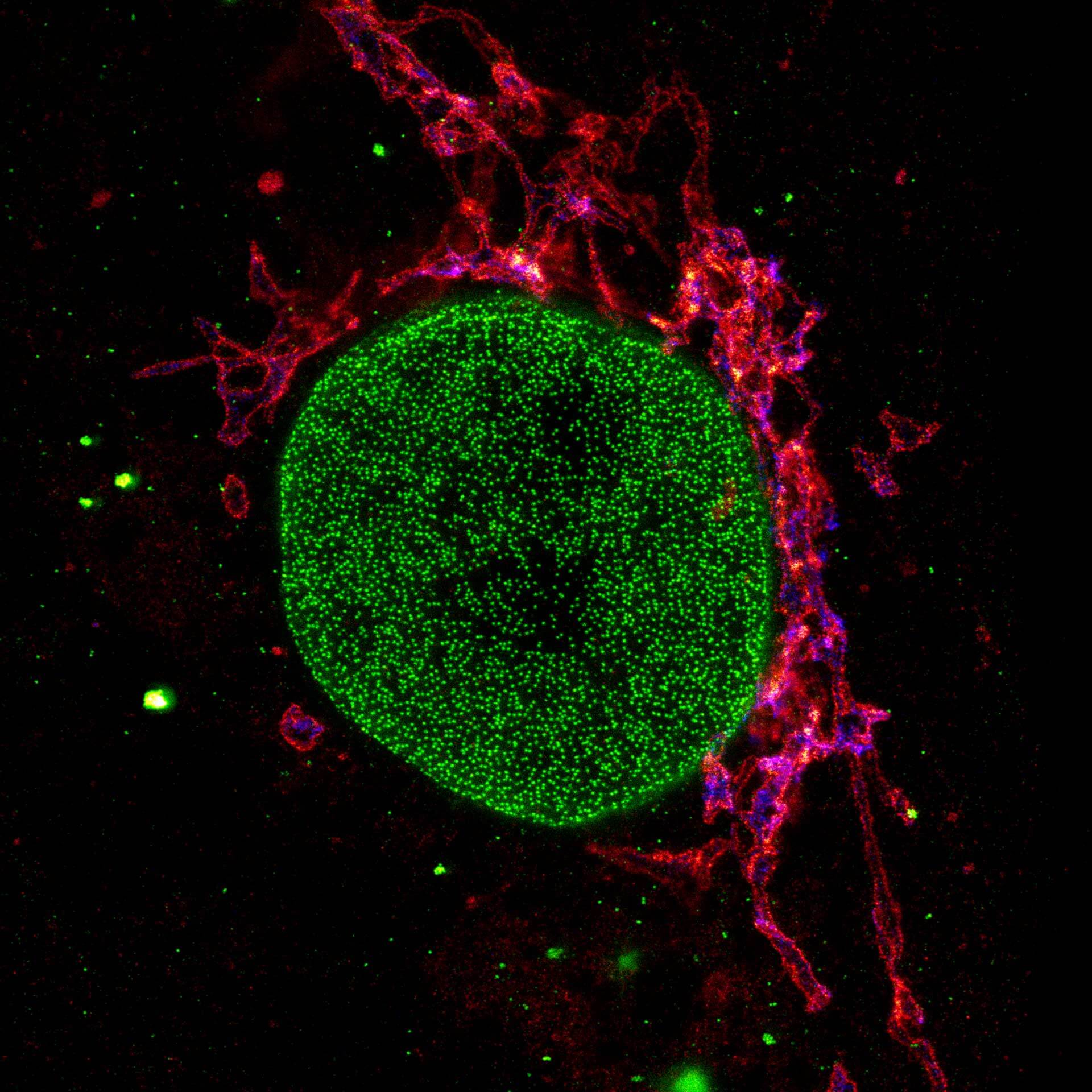
Description
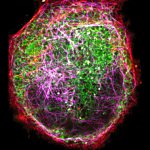
Three color STED and confocal image of a mammalian cultured cell immunostained for a nuclear pore protein in green and two golgi apparatus markers in red and blue.
abberior STAR RED highlights the golgi apparatus protein giantin (red) and abberior STAR ORANGE visualizes the cis-golgi protein GM130 (blue). NUP98 proteins were stained with abberior STAR GREEN (green).
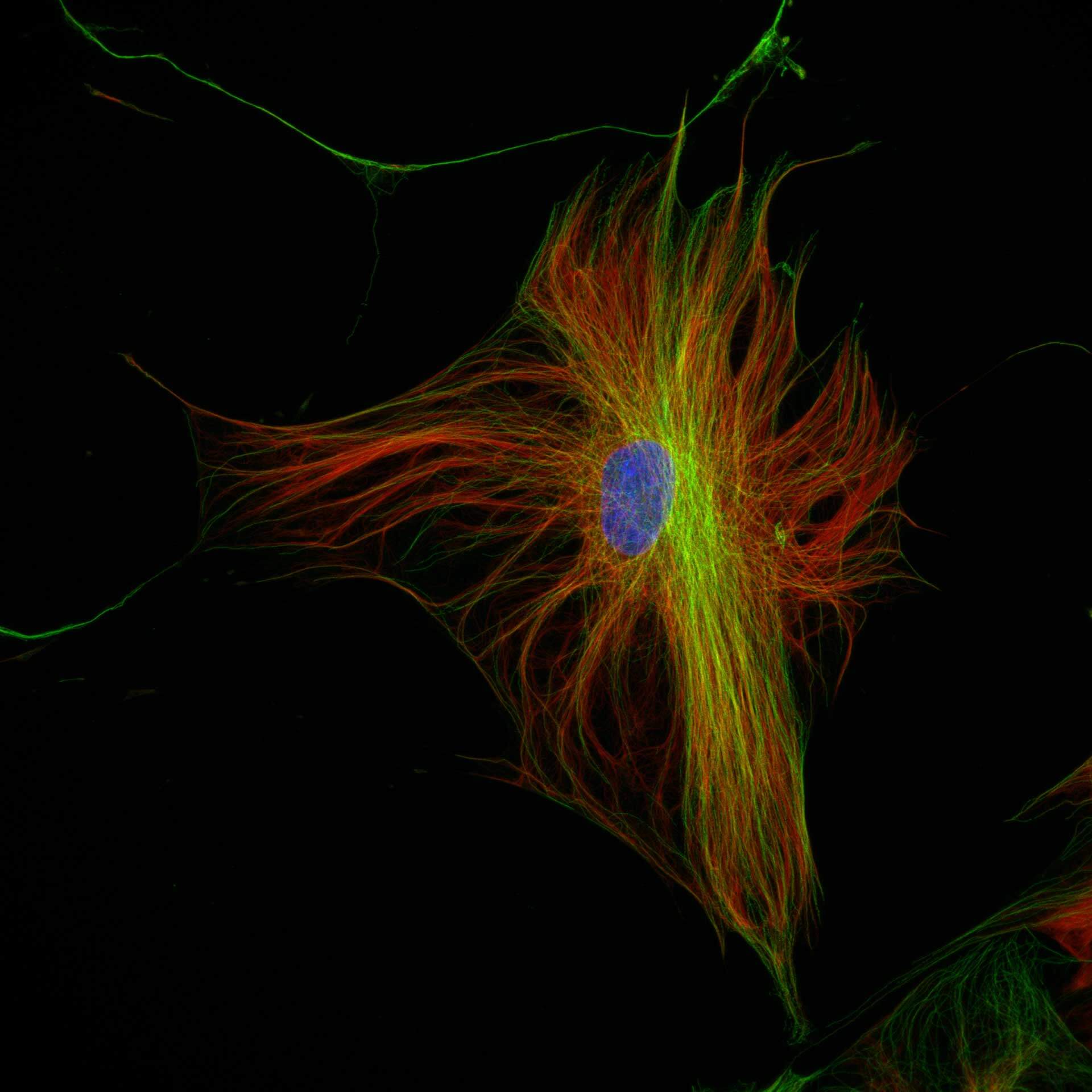
Description
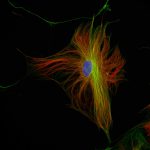
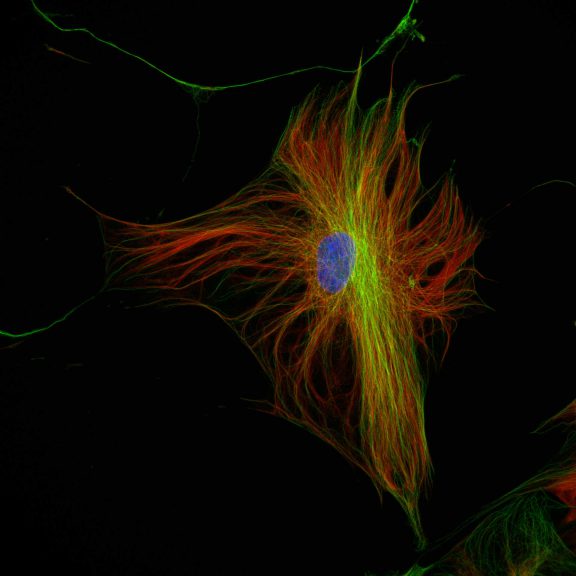
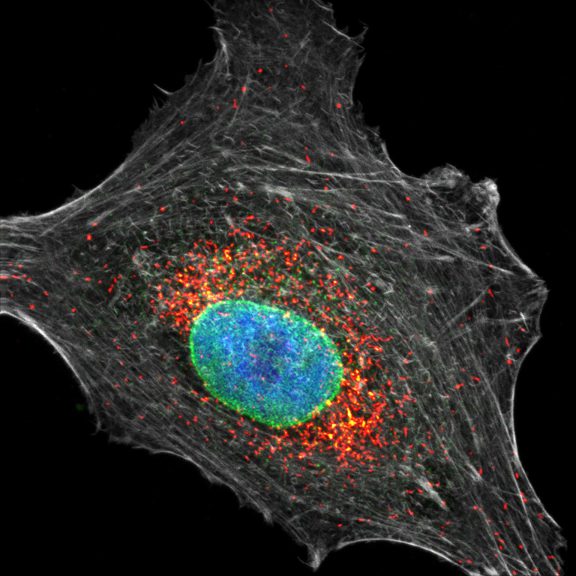
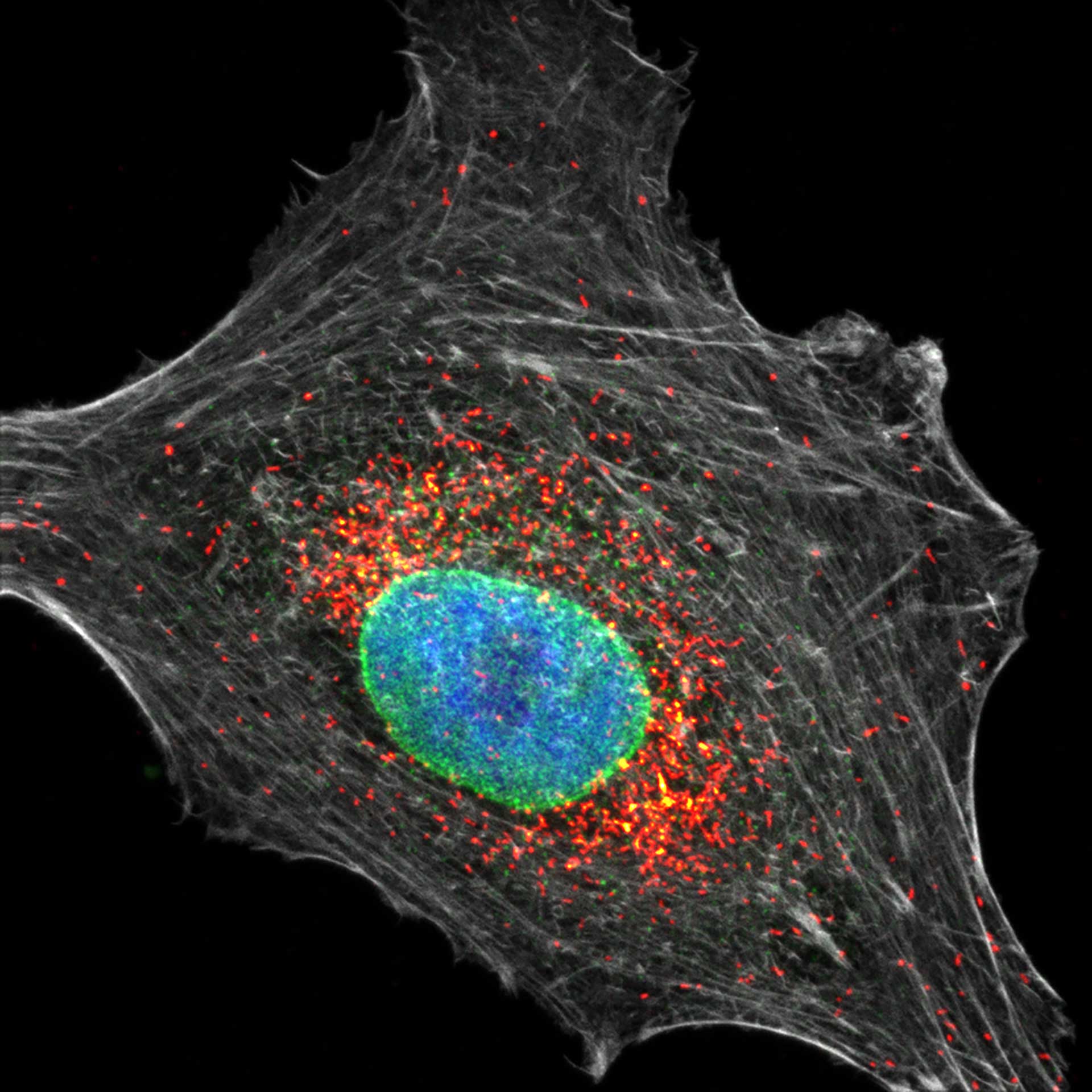
Three color confocal image of human fibroblast immunostained with abberior STAR 580 for vimentin (green) and with abberior STAR RED for tubulin (red).
DAPI was added to highlight the nucleus (blue).
Description
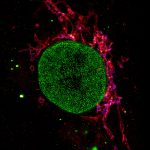
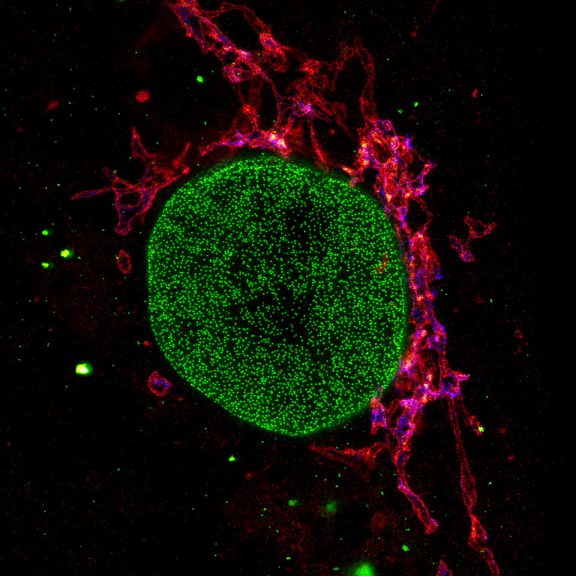
Four color confocal image of mammalian cultured cells. Peroxisomes were immunostained with abberior STAR RED (magenta) and nuclear pore proteins with abberior STAR 580 (green). F-actin fibers were highlighted with abberior STAR 488 phalloidin (gray).
The nucleus was visualized with DAPI (blue).
Description
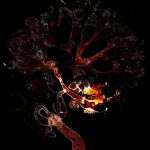
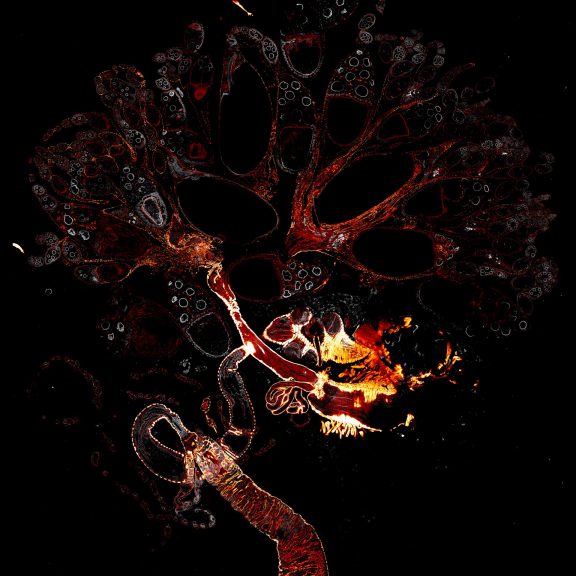
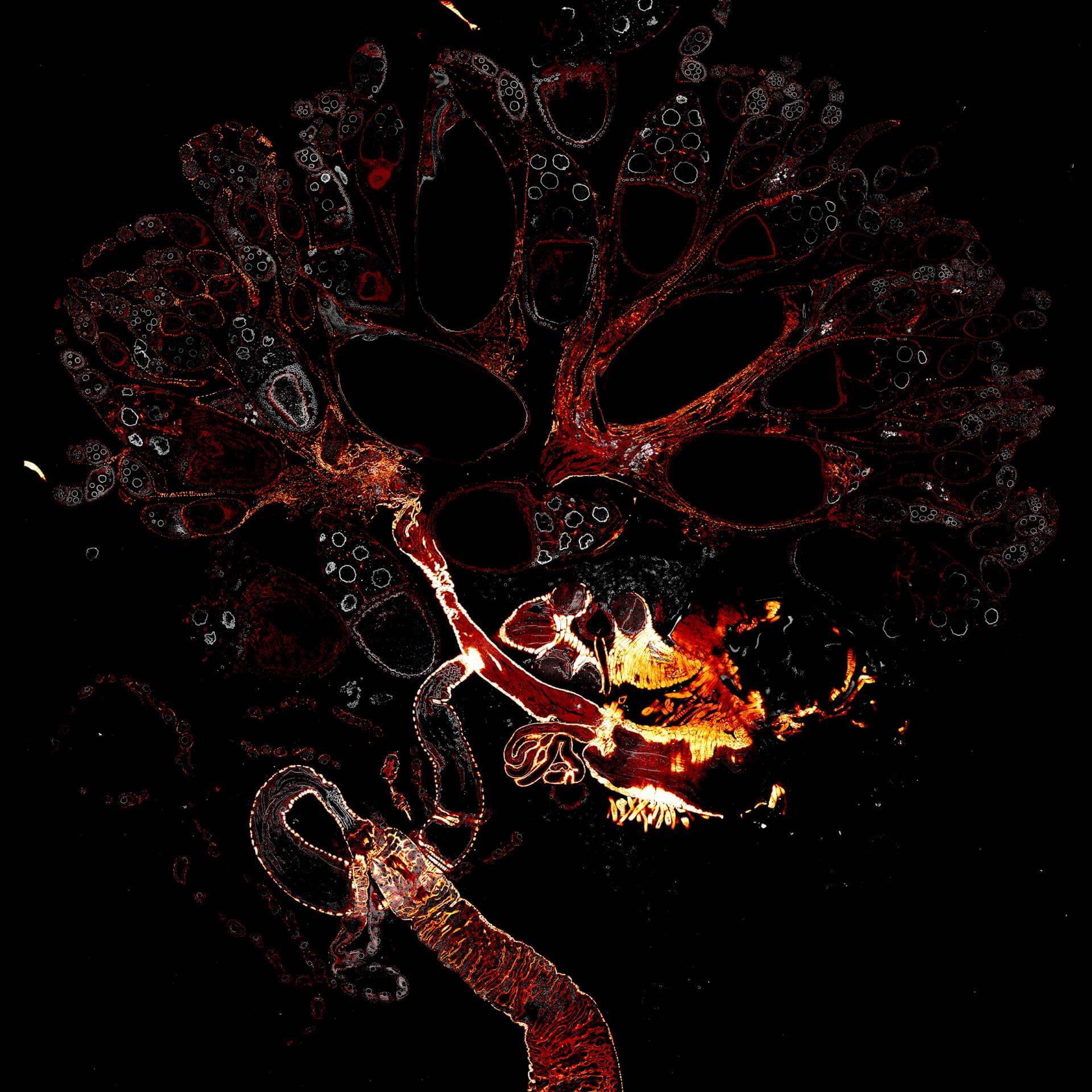
Drosophila female reproductive system stained for F-actin (red) with abberior STAR RED phalloidin. abberior STAR ORANGE is highlighting a nuclear pore protein (gray).
Image was acquired with the STEDYCON tiling feature and assembled with the SVI Huygens Stitcher.
Description
MINFLUX imaging of βII spectrin in a primary hippocampal neuron labeled with abberior FLUX 680 by indirect immunofluorescence. Please note the periodic arrangement of spectrin along the axon.
Description
MINFLUX image of axonal βII spectrin labeled with abberior FLUX 660 in primary hippocampal neurons. Note the periodic arrangement of spectrin along the axon, and the absence of any details in the confocal counterpart image.
Description
2D MINFLUX nanoscopy of the nuclear pore complex subunits. Fixed mammalian cells expressing SNAP-tag® NUP96 were labeled with abberior FLUX 647 SNAP. In contrast to confocal microscopy, 2D MINFLUX allows to visualize the shape and arrangement of individual subunits of the nuclear pore complex.
Description
2D MINFLUX imaging of the peroxisomal membrane protein PMP70 labeled with abberior FLUX 640 in fixed mammalian cells using indirect immunofluorescence.
Description
Drosophila ovariole stained with abberior LIVE 560 DNA showing nuclei in different cell types of the egg chamber. Ovaries were dissected from adult female fruit flies and were fixed prior to staining.
Image was acquired with the STEDYCON tiling feature and assembled with the SVI Huygens Stitcher.
Description
Drosophila spermatid tails were stained with abberior LIVE 610 tubulin. Testis were dissected from adult male fruit flies. Live cell imaging experiment was performed on a INFINITY microscope.
Description
Composite of three color live-cell image of an adherent mammalian cell. This living cell was directly labelled with abberior LIVE 510 actin (blue/green), LIVE 560 DNA (gray) and LIVE 610 tubulin. This image was acquired with a STEDYCON microscope.
Description
Three color live-cell STED at 775 nm: living cell labelled with abberior LIVE 460L (ER, green), LIVE 560 tubulin (magenta) and LIVE 610 actin (red).
Description
Confocal and STED time lapse of a living cell expressing SNAP-tag® fusion protein fusion protein in the endoplasmic reticulum lumen with SNAP and C-terminal tetrapeptide KDEL. SNAP-KDEL was labelled with abberior LIVE 610 SNAP ligand (benzylguanine).
The SNAP-KDEL plasmid was a gift from Francesca Bottanelli, FU Berlin.
Description
Live cell time lapse of abberior LIVE 590 DNA probe in cultured mammalian cells.
The use of very low concentrations of our abberior LIVE dyes reduces toxicity and allows long-term imaging of dynamic processes.
Image was acquired with the FACILITY microscope.
Description
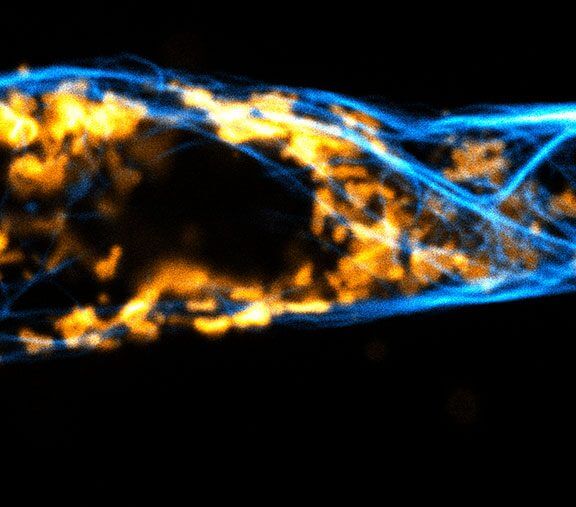
Two color live-cell confocal and STED image of a mammalian cell expressing a SNAP-tag® OMP25 fusion protein decoration the outer membrane of mitochondria. OMP25 is visualized by our new abberior LIVE 610 SNAP ligand (orange). Tubulin filaments are highlighted with abberior LIVE 550 tubulin (cyan).
The SNAP-OMP25 plasmid was a gift from Francesca Bottanelli, FU Berlin.
Description
Two color live-cell confocal and STED image of a mammalian cell expressing a SNAP-tag® OMP25 fusion protein decoration the outer membrane of mitochondria. OMP25 is visualized by our new abberior LIVE 610 SNAP ligand (orange). Tubulin filaments are highlighted with abberior LIVE 550 tubulin (cyan).
The SNAP-OMP25 plasmid was a gift from Francesca Bottanelli, FU Berlin.
Description
Cultured mammalian cell immunostained for an inner and outer mitochondrial membrane marker. Outer membrane is highlighted with abberior STAR RED (red) and the inner membrane with abberior STAR ORANGE (gray).
STED and confocal images were acquired with abberior's FACILITY microscope.
Description
Drosophila male accessory gland stained for F-actin using abberior STAR 580 phalloidin.
Sample was prepared in cooperation with Dr. H. R. Shcherbata at MPl for Biophysical Chemistry, Göttingen, Germany.
Description
Three color STED and confocal image of a mammalian cultured cell immunostained for a nuclear pore protein in green and two golgi apparatus markers in red and blue.
abberior STAR RED highlights the golgi apparatus protein giantin (red) and abberior STAR ORANGE visualizes the cis-golgi protein GM130 (blue). NUP98 proteins were stained with abberior STAR GREEN (green).
Description
Three color confocal image of human fibroblast immunostained with abberior STAR 580 for vimentin (green) and with abberior STAR RED for tubulin (red).
DAPI was added to highlight the nucleus (blue).
Description
Four color confocal image of mammalian cultured cells. Peroxisomes were immunostained with abberior STAR RED (magenta) and nuclear pore proteins with abberior STAR 580 (green). F-actin fibers were highlighted with abberior STAR 488 phalloidin (gray).
The nucleus was visualized with DAPI (blue).
Description
Drosophila female reproductive system stained for F-actin (red) with abberior STAR RED phalloidin. abberior STAR ORANGE is highlighting a nuclear pore protein (gray).
Image was acquired with the STEDYCON tiling feature and assembled with the SVI Huygens Stitcher.