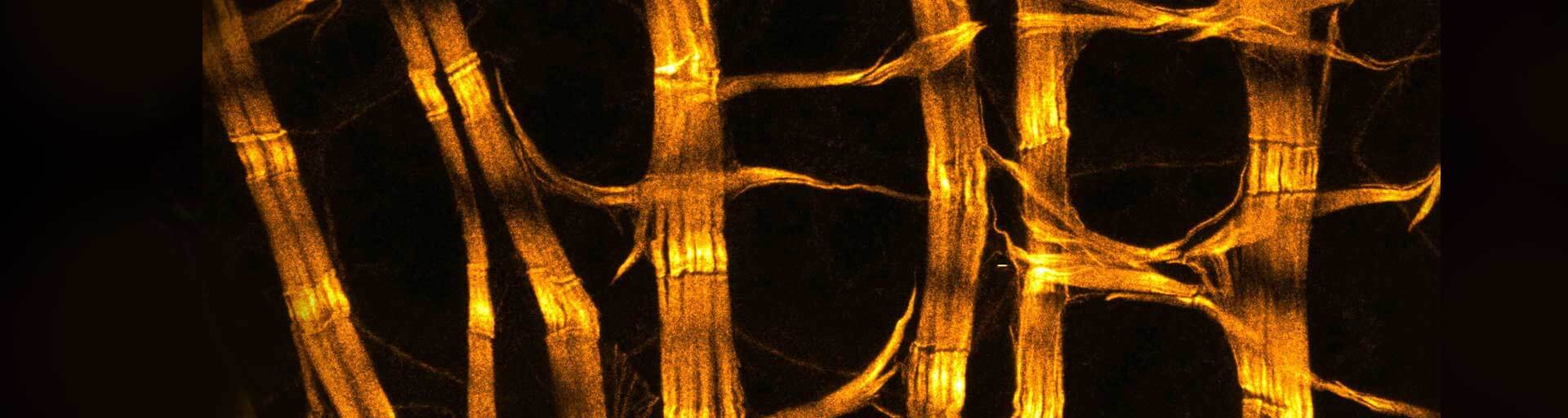
Phalloidin labeling protocol
Fluorescent phalloidin conjugates can be used to stain actin filaments (F-actin) in fixed specimens, making it an essential tool to visualize the actin skeleton of cultured cells, tissue, whole organs, or plants.
for easy and proper application of our labels
Introduction
abberior offers a variety of excellent fluorescent dyes with properties optimized for the labeling of biomolecules, spectroscopic studies, and optical microscopy, particularly super-resolution microscopy, and optical nanoscopy.
Phalloidin belongs to the class of phallotoxins found in Amanita phalloides. Fluorescent phalloidin conjugates can be used to stain actin filaments (F-actin) in fixed specimens, making it an essential tool to visualize the actin skeleton of cultured cells, tissue, whole organs, or plants. Due to its binding affinity and very high specificity, phalloidin is the ideal probe for microscopy applications.
Note: Phalloidin is a toxic substance (LD50 toxicity of 2 mg/kg), please refer to the Material Safety Data Sheet (MSDS) provided as a download in our web-shop. All phalloidin conjugates must be used by, or under the direct supervision of, technically qualified individuals.
Storage
Our abberior phalloidin probes are freeze-dried and shipped at room temperature. Upon arrival, the product can be stored for up to one year at –20 °C. Shortly before the staining procedure dissolve the probe in DMF or DMSO. Once dissolved the stock solutions should be kept at –20 °C, protected from light and moisture.
Note: Depending on solvent quality the shelf-life of the stock solutions might be significantly reduced compared to the phalloidin conjugate in its solid form. Repeated freeze-thaw cycles can be avoided by splitting the dissolved compound into smaller aliquots.
Preparing the Stock Solution
The product contains a vial with 20 µg lyophilized powder of the abberior phalloidin conjugate.
The powder should be dissolved in DMF or DMSO to yield a final concentration of 200 Units/ml, which is equivalent to approximately 6.6 µM = 6.6 µmol/l = 6.6 nmol/ml. For calculation please use the following formula:
\(\frac{20\ \text{μg phalloidin conjugate} }{\text{Molecular weight phalloidin conjugate (µg/µmol)} \times 6.6\ \text{µmol/l} } =\text{Volumen solvent (l)}\)
Note: The molecular weight may differ for each conjugate and the relevant molecular weight can be found on the shipping pouch or in our web-shop.
Labeling with abberior phalloidin conjugates
The two procedures below have been successfully tested with our abberior STAR dye conjugated to phalloidin. These procedures have yielded consistent results in most instances but may require further optimization for particular model organisms. The following two protocols describe the staining procedure for adherent cells grown on glass coverslips and whole-mount Drosophila tissue samples.
Required reagents; not provided
- glass coverslips, glass-bottom dish, or similar imaging chamber with a glass thickness of ~170 µm (No. 1.5 or No. 1.5H)
Note: We do not recommend using plastic coverslips or live-cell chambers with plastic bottoms because frequently only suboptimal imaging results are achieved. If possible, coverslips with grids, gratings, or similar should be avoided, as these structures can interfere with imaging causing aberrations that degrade image quality - 1x Phosphate-buffered saline pH 7,4 (PBS)
- 2% – 8% methanol-free Formaldehyde in PBS pH 7,4 (PFA)
- 0,1% – 0,5% Triton X-100 in PBS pH 7,4
- 1% – 3% Bovine Serum Albumin + 0,1% Tween20 in PBS pH 7,4 (PBT)
- Mounting Medium
- Tweezers
One Unit/ml of phalloidin conjugate is defined as the amount of material used to stain one microscope slide of fixed cells or tissue, according to the following protocols.
Staining procedure for cultured cells
All steps are carried out at room temperature.
- Fix cells with PFA. Depending on the cell type, fixation will take between 5 min to 15 min.
- Discard the fixation solution and extract the cells with Triton-X 100 for 5 min to 15 min.
- Wash the cell three times with PBS.
- Finally, unspecific binding sides are blocked with PBT for 30 min to 1 h in a petri dish.
- Take the cover slips out of the petri dish; remove excess PBT by placing the cover slip edge onto a piece of tissue paper. Transfer the coverslips to a humid chamber, cells facing upwards. Add the phalloidin staining solution (1 Unit/ml) onto the coverslips and incubate it for 1 h in the humid chamber.
- Fill a petri dish with PBS, until the bottom is covered with enough liquid. Transfer the coverslip back into the petri dish, cells facing upwards.
- Rinse the cells one more time with PBS. Remove excess PBS by placing the cover slip edge onto a piece of tissue paper.
- Mount the coverslip with a suitable mounting medium.
Note: Phalloidin stain can be combined with immunostaining. Therefore, the phalloidin conjugate can be added either to the primary or secondary antibody staining solution. This procedure can be combined with your standard immunostaining protocol.
Staining procedure for whole-mount Drosophila tissue samples
All steps are carried out at room temperature on a rotator.
- Fix the sample with PFA containing 1 Unit/ml of the phalloidin conjugate; fixation will take between 15 min to 30 min depending on your tissue sample.
- Discard the fixation solution and extract the sample with Triton-X 100 containing 1 Unit/ml of the phalloidin conjugate for 15 min to 20 min.
- Repeat step 2. two more times.
- Wash the sample three times with PBS for 15 min to 20 min each.
- Remove excess PBS.
- Mount the sample with a suitable mounting medium.
Note: Phalloidin stain can be combined with immunostaining. Please use your standard immunostaining protocol after step 2.
Abbreviations
DMF N,N-Dimethylformamid
DMSO Dimethylsulfoxide
PBS Phosphate-buffered saline
PFA Paraformaldehyde
PBT 1% – 3% Bovine Serum Albumin + 0.1% Tween20 in PBS
min Minute
h Hour