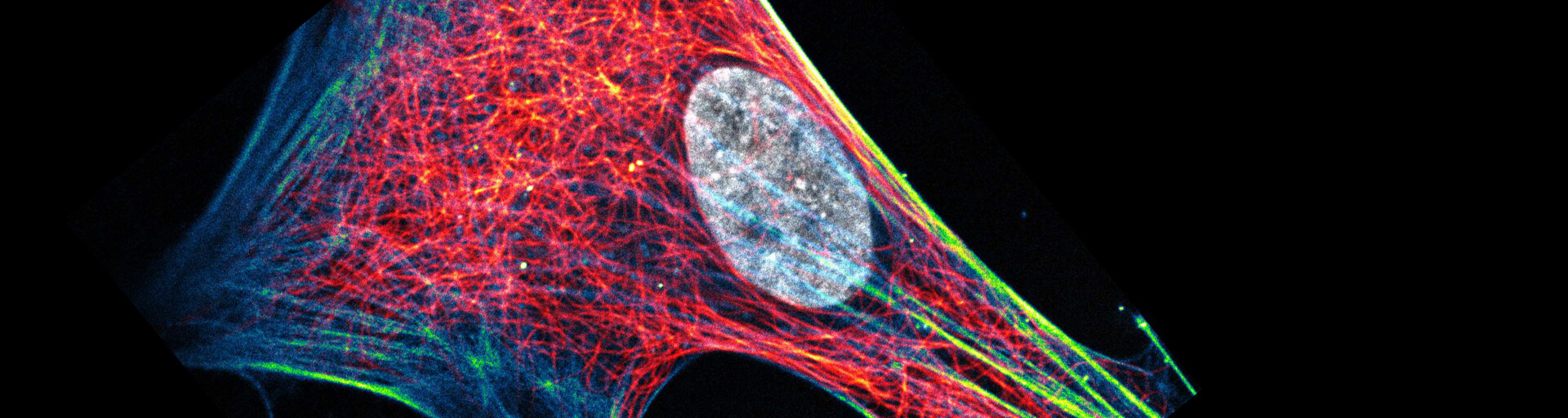
Live cell protocol
Our abberior LIVE labels can be readily used to specifically label microtubule filaments, actin network, or double-stranded DNA in living cells or can be used for in vitro studies.
for Tubulin, Actin and DNA in living specimens
Introduction
abberior offers a variety of excellent fluorescent dyes with properties optimized for the labeling of biomolecules in living specimens, spectroscopic studies, and, optical microscopy, particularly STED super-resolution microscopy. These unique dyes combine the highest brightness and photostability with easy live cell labeling.
Our abberior LIVE labels can be readily used to specifically label microtubule filaments, actin network, or double-stranded DNA in living cells or can be used for in vitro studies. In addition, due to the beneficial properties of our unique abberior LIVE labels, minimal amounts can be used for obtaining fully labeled structures reducing potential toxic effects and cellular stress.
To all our LIVE labels one vial of Verapamil is added. Some cell types actively pump out the probes via efflux pumps located at the membrane of the cells. Since the probes are not covalently bound to the target they can be washed out by those pumps. This can cause dimmer fluorescence signals which may decrease further during long-term imaging. As a countermeasure, the efflux pump inhibitor Verapamil can be applied in the staining solution.
Storage
abberior LIVE labels and Verapamil are shipped freeze-dried in amounts of 50 µg per vial (or 2 nmol in case of evaluation samples) and 1 µmol, respectively. Upon arrival, the substances can be stored for up to one year at –20 °C. Shortly before the staining procedure dissolve the probe and Verapamil in DMF or DMSO. Once dissolved the stock solutions should be kept at –20 °C, protected from light and moisture.
Note: Depending on solvent quality the shelf-life of the stock solutions might be significantly reduced compared to the substances in their solid form – even if stored at –20 °C.
Staining of intracellular targets using abberior LIVE tubulin, actin, and DNA labels
The procedure described below has been successfully tested with our abberior LIVE labels for direct labeling in several adherent mammalian cultured cell lines and Drosophila melanogaster dissected organs. This procedure has yielded consistent results in most instances but may require further optimization for particular model organisms.
Required reagents / equipment; not provided
- DMF or DMSO
- Glass coverslips, glass-bottom dish, or similar imaging chamber with a glass thickness of ~170 µm (No. 1.5 or No. 1.5H)
Note: We do not recommend using plastic coverslips or live-cell chambers with plastic bottoms because frequently only suboptimal imaging results are achieved. If possible, coverslips with grids, gratings, or similar should be avoided, as these structures can interfere with imaging causing aberrations that degrade image quality - Live-cell imaging medium
- Optional: Cavity slide or coverslip holder
- Optional: silicone glue (e. g. Twinsil, Picodent)
- Fluorescence microscope with live-cell incubator, suitable excitation light source, and detection filter
Staining procedure for cultured cells
The seeding time should be adjusted according to the doubling time of the particular cell line. Seed cells at the desired density in a cell culture chamber or onto coverslips before labeling. Use a standard cell culture medium that is optimal for the cell line.
Note: Cells grown in very high densities, i.e., a confluent layer, may give rise to high labeling background.
Staining and imaging will take place on the same day
- Prewarm the live-cell imaging medium to the optimal temperature required to cultivate the desired cell line. In most cases, this is 37 °C.
- Please dissolve the probe in DMF or DMSO for preparing a dye stock solution with a concentration of 1 mM. The molecular weights of abberior LIVE labels are given for each product individually on the product pouches as well as on the abberior web-shop.
- Prepare the staining solutions with final dye concentrations of 0.01 to 1 µM. For this, the stock solution of the abberior LIVE labels is further diluted in a pre-warmed live-cell imaging medium.
Note: The concentration required for proper labeling strongly depends on the specimen (tissue or cell). It can vary drastically between cell types and experimental conditions.
Note: Staining solutions are not stable for extended periods of time. Therefore, it is recommended that you only prepare enough solution for immediate use. After a few days, probes may form aggregates in aqueous solutions which may reduce labeling efficiency.
Optional: Add dissolved Verapamil stock solution to the staining solution. Dilute Verapamil stock solution to give a final concentration between 1 and 25 µM.
Optional: Our direct LIVE labels can be combined with our membrane probes. Simply add them to the staining solution for multicolor live-cell imaging.
- Remove the cell culture medium and rinse the cells once with a prewarmed live-cell imaging medium.
- Remove the live-cell imaging medium and add a sufficient amount of staining solution to the cells. Incubate for 30 to 60 min at optimal cell growth conditions (temperature, humidity, CO2-controlled environment).
abberior LIVE probes are used in very low nanomolar concentration, meaning the background signal is already reduced. Consequently, a washing step is not required but can be done optionally – for washing cells can be rinsed with fresh live-cell imaging medium.
Note: Often, the cell benefit from a reduction of the probe concentration instead of incubating them longer in the staining solution (e. g. 16 to 24 h). - Remove the staining solution.
If using cells grown on coverslips: Simply take the coverslip out of the staining solution using tweezers. Place the coverslip in a coverslip holder and fill it with fresh live cell imaging medium. Alternatively, mount the coverslip (cells facing downwards) onto a slide with a cavity filled with fresh live-cell imaging medium.
In case of keeping/imaging cells in a cavity slide, remove any excess imaging medium using tissue paper. Gently press down the coverslip to prevent it from moving. The mounted sample can be sealed using silicone glue (e. g. Twinsil, Picodent).
Note: Depending on the sample, it can be advantageous to add one-tenth of the staining solution of abberior LIVE dye probe in the embedding medium (v/v). This is especially the case for long imaging times. - After staining and mounting, the samples should be imaged directly on a microscope equipped with a live-cell incubator.
Note: For live-cell imaging, cells must always be kept at ambient conditions (temperature, humidity, pH, and CO2-conditions). This is particularly important for long-term measurements.
Abbreviations
DMF N,N-Dimethylformamid
DMSO Dimethylsulfoxide
STED Stimulated Emission Depletion