Premix & stain protocol
For very fast and easy labeling, premix a primary antibody with our abberior nanobody-conjugates and stain your biological sample in just a few steps.
for easy and proper application of our secondary nanobodies
Introduction
abberior offers a variety of excellent fluorescent dyes with properties optimized for labeling biomolecules, spectroscopic studies, and optical microscopy, particularly super-resolution microscopy, and optical nanoscopy.
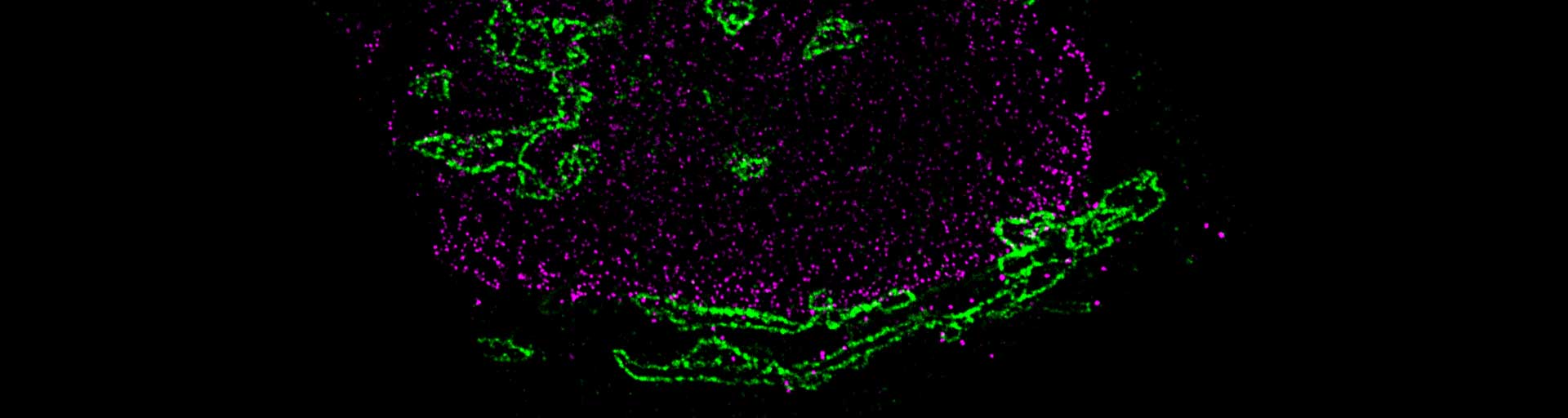
The combination of super bright and highly photostable abberior dyes and the polyclonal nanobodies produced in alpacas from Jackson ImmunoResearch (AffiniPure-VHH™ Secondaries) offer a fantastic solution for high-resolution imaging experiments where small labels, good penetration, and outstanding specificity are necessary.
Storage
The product contains a vial of nanobody-conjugate in solution and is shipped at room temperature. Upon arrival, the product can be stored at 4 °C for a short period of time. For long-term storage of up to one year add an equal volume of glycerol (ACS grade or better) for a final concentration of 50 %, and store at –20 °C as a liquid. The conjugate should be protected from direct light exposure. Repeated freeze-thaw cycles should be avoided. Therefore, it is recommended to split the abberior secondary nanobody solution into smaller aliquots.
Pemix & stain procedure
The procedure has been successfully tested with our abberior dye conjugates for adherent cells grown on glass coverslips and has yielded consistent results in most instances but may require further optimization for particular model organisms or experimental conditions.
The success of the premix & stain protocol depends strongly on the primary antibody. The affinity to the target protein can be drastically reduced if an antibody-nanobody complex is formed. However, this should be tested individually.
Required reagents; not provided
- Phosphate-buffered saline pH 7.4 (PBS)
- Fixative depending on the primary antibody
- 0.1% – 0.5% Triton X-100 in PBS pH 7.4 (permeabilization buffer)
- 1% – 3% Bovine Serum Albumin + 0.1% Tween20 in PBS pH 7.4 (blocking buffer, PBT)
- Primary antibody
- Mounting Medium
- Glass coverslips with a glass thickness of ~170 µm (No. 1.5 or No. 1.5H)
Note: We do not recommend using plastic coverslips because frequently only suboptimal imaging results are achieved. If possible, coverslips with grids, gratings, or similar should be avoided, as these structures can interfere with imaging causing aberrations that degrade image quality. - Humid chamber
- Fluorescence microscope with suitable excitation light source and detection filter
Premix & stain procedure for cultured cells (single color staining)
All steps are carried out at room temperature and in a petri dish unless stated otherwise.
- Incubate cells with a fixative suitable for the primary antibody in use for 5 min to 15 min.
Note: When using Methanol as a fixative, step 2 can be skipped. - Replace the medium with permeabilization buffer and incubate the sample for 5 min to 15 min.
- Wash cells 3 x 5 min in PBS.
- Replace the medium with a blocking buffer and incubate the sample for 30 min to 60 min.
- Add primary antibody and secondary nanobody to a fresh reaction tube in a ratio of 1.0 µg primary antibody to 0.3 – 1.0 µg secondary nanobody and fill the solution with PBS (pH 7.4) until a maximum volume of 20 µl is reached.
- Incubate the antibody-nanobody solution for 20 to 40 min; dilute the antibody-nanobody complex to the final staining volume in PBS (ca. 100 µl per 1 µg primary antibody and coverslip).
Note: In this step, BSA or any other serum should be avoided, as the nanobodies can bind to free IgG antibodies within the serum and thus preventing optimal staining.
Note: The ratio of primary antibody to secondary nanobody depends on factors such as the target protein/structure, dye, and primary antibody. The ideal ratio must therefore be determined individually for each experiment. - Take the cover slips out of the petri dish; remove the excess blocking buffer by placing the cover slip edge onto a piece of tissue paper. Transfer the coverslips into a humid chamber, cells facing upwards. Add the antibody-nanobody complex onto the coverslips and incubate for 20 min to 60 min in the humid chamber under the exclusion of light.
- Remove excess antibody-nanobody complex by placing the cover slip edge onto a piece of tissue paper. Wash the cells in PBS (3 x 5 min) using a fresh petri dish.
- Take the cover slip out of the washing solution, remove excess PBS by placing the cover slip edge onto a piece of tissue paper, and mount the coverslip with a suited mounting medium.
Abbreviations
PBS Phosphate-buffered saline
PFA Paraformaldehyde
PBT 1% – 3% Bovine Serum Albumin + 0.1% Tween20 in PBS
min Minute