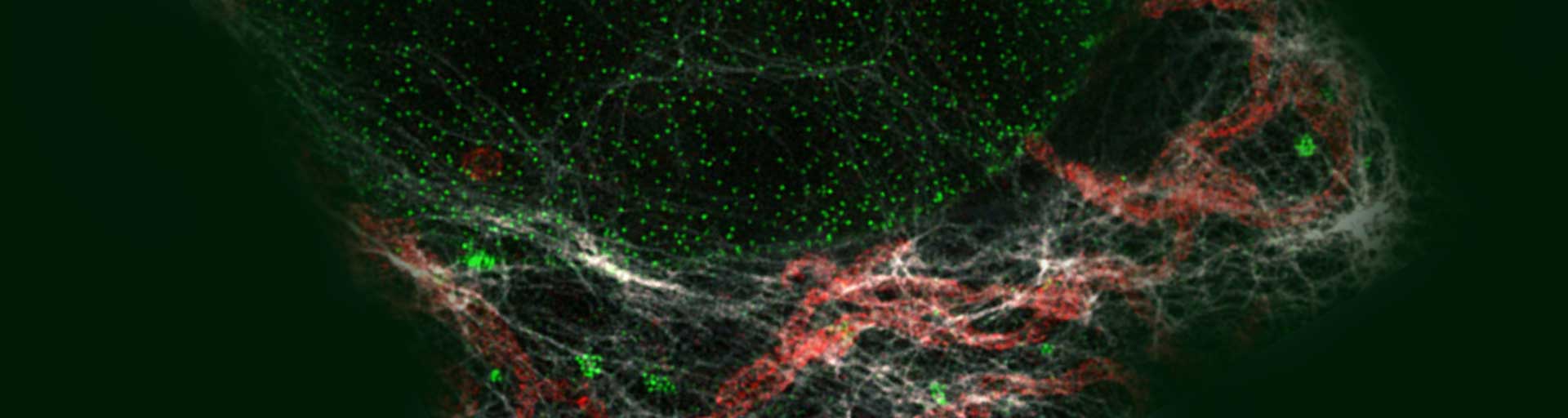
Immunolabeling protocol
Our abberior STAR dyes conjugated with secondary antibodies are designed for the best performance in STED and confocal microscopy. They feature high brightness and photostability, especially in the red and infrared wavelength ranges. These fluorescent dyes are tailor-made for superresolution nanoscopy and guarantee sharp and clear images and experimental success.
for easy and proper application of our labels
Introduction
abberior offers a variety of excellent fluorescent dyes with properties optimized for the labeling of biomolecules, spectroscopic studies, and optical microscopy, particularly super-resolution microscopy, and optical nanoscopy.
Our abberior fluorescent secondary antibodies are also relevant to other techniques that rely on the use of fluorophore-conjugated antibodies such as flow cytometry, ELISA, western blot, and immunohistochemistry.
Storage
The product contains a vial of buffered solution and is shipped at room temperature. Upon arrival, the product can be stored at 4 °C for up to one month or at –20 °C to –80 °C for long-term storage of up to one year. The conjugate should be protected from direct light exposure and repeated freeze-thaw cycles should be avoided. For this, it is recommended to split the abberior secondary antibody solution into smaller aliquots.
Indirect immunofluorescence staining with abberior secondary antibodies
The two procedures below have been successfully tested with our abberior dye conjugates. These procedures have yielded consistent results in most instances but may require further optimization for particular model organisms. The following two protocols describe the staining procedure for adherent cells grown on glass coverslips and whole-mount Drosophila melanogaster tissue samples.
Required reagents; not provided
- 1x Phosphate-buffered saline pH 7.4 (PBS)
Fixative depending on primary antibody:
- 2% – 8% Formaldehyde in PBS pH 7.4 (PFA)
- Methanol (abs.)
- 4% PFA + 0.02% Glutaraldehyde in PBS pH 7.4
- 0.1% – 0.5% Triton X-100 in PBS pH 7,4
- 1% – 3% Bovine Serum Albumin + 0.1% Tween20 in PBS pH 7.4 (PBT)
- Primary antibody
- Mounting Media
Staining procedure for cultured cells
All steps are carried out at room temperature in a petri dish unless stated otherwise.
- Fix cells with a fixative suitable for the primary antibody in use. Depending on the cell type, fixation will take between 5 min to 15 min.
Note: When using Methanol as a fixative, step 2 can be skipped. - Discard the fixation solution and extract the cells with Triton-X 100 for 5 min to 15 min.
- Wash the cell three times with PBS for 5 min.
- Incubate the sample in PBT for 30 min to 1 h to block unspecific binding sides.
- Dilute the primary antibody to the final working concentration in PBT.
- Take the coverslips out of the petri dish; remove excess PBT by placing the coverslip edge onto a piece of tissue paper. Transfer the coverslips into a humid chamber, cells facing upwards. Add the primary antibody onto the coverslips and incubate for 1 h in the humid chamber.
- Wash the samples in PBS (3 x 5 Minutes) using a fresh petri dish.
- Wash the cells with Triton-X 100 for 5 min.
- Incubate the sample in PBT for 30 min to 1 h.
- Dilute the secondary antibody to the final staining/working concentration in PBT.
Note: abberior secondary antibody conjugates have a concentration of 1 mg/ml. For most applications, a dilution of 1:200 (5 µg/ml) is sufficient. However, staining protocols may vary with cell type and application.
Optional: At this step abberior phalloidin can be added to the staining solution (1 Unit/ml). - Take the cover slips out of the petri dish; remove excess PBT by placing the cover slip edge onto a piece of tissue paper. Transfer the coverslips into a humid chamber, cells facing upwards. Add the secondary antibody onto the coverslips and incubate for 1 hr in the humid chamber.
- Wash the cells in PBS (3 x 5 Minutes) using a fresh petri dish.
- Take the coverslip out of the washing solution, remove excess PBS by placing the coverslip edge onto a piece of tissue paper, and mount the coverslip with a suited mounting media.
Staining procedure for whole-mount Drosophila tissue samples
All steps are carried out at room temperature unless stated otherwise. Tissue samples are placed within a tube on a rotator.
- Fix the dissected tissue with a fixative suitable for the primary antibody in use. Depending on the thickness of the sample, fixation will take between 15 min to 30 min.
- Discard the fixation solution and wash the sample three times with Triton X-100 for 15 min.
- Incubate the sample in PBT for 1 h.
- Dilute the primary antibody to the final working concentration in PBT.
- Remove excess PBT and add the primary antibody into the tube. Incubate the sample overnight at 4 °C on a rotator.
- Remove the primary antibody solution from the tube and wash the tissue sample three times with Triton X-100 for 15 min.
- Incubate the sample in PBT for 1 h.
- Dilute the secondary antibody to the final working concentration in PBT.
Note: abberior secondary antibody conjugates have a concentration of 1 mg/ml. For most applications, a dilution of 1:200 (5 µg/ml) is sufficient. However, staining protocols may vary with cell type and application.
Optional: At this step abberior phalloidin can be added to the staining solution (1 Unit/ml). For thick samples or whole organs, please refer to our phalloidin protocol. - Remove excess PBT and add the secondary antibody into the tube. Incubate the tissue sample 3 h to 4 h at room temperature on a rotator.
- Remove the secondary antibody solution and wash the tissue sample three times with PBS for 15 min.
- Mount the tissue sample with a suited mounting medium.
Abbreviations
STED Stimulated Emission Depletion
PBS Phosphate-buffered saline
PFA Paraformaldehyde
PBT 1% – 3% Bovine Serum Albumin + 0.1% Tween20 in PBS
min Minute
h Hour