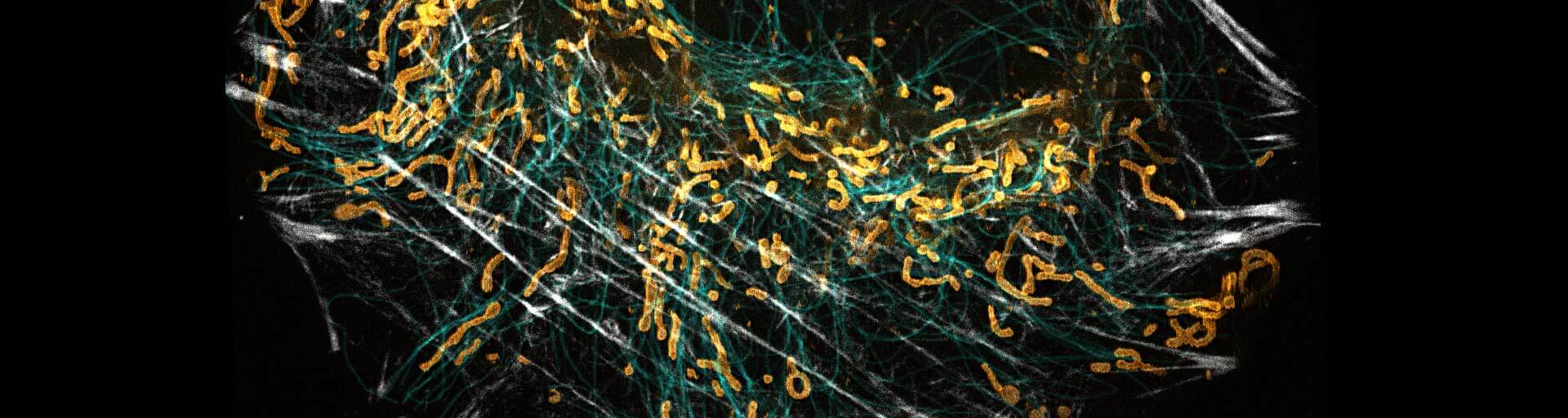
Labeling Protocol for HaloTag®
Your HaloTag® fusion proteins within living cells, on cell surfaces, and on fixed cells can be reversibly colored with the abberior LIVE HaloX labels. In a PAINT-like process imaging of the sample in an extended period of time is easily possible, as bleaching is no longer a decisive factor.
with abberior LIVE HaloX® labels
Introduction
abberior offers a variety of excellent fluorescent probes with properties optimized for the labeling of biomolecules in living specimens, for spectroscopic studies, and for optical microscopy, particularly STED superresolution microscopy. These unique probes combine the highest brightness and photostability with easy live-cell labeling.
abberior’s HaloX®1 labels2 can be readily used to specifically label your HaloTag®3 fusion proteins of interest within living cells or on cell surfaces. Due to high affinity, the probes interact with the HaloTag® protein but can exchange regularly. The PAINT-like process ensures that proteins can be detected in the living cell for extended periods of time, as bleaching processes are no longer a decisive factor. This is possible by replacing the chloride of the conventional HaloTag® ligand with a sulfonamide, which ensures that no enzymatic reaction can take place between the probes and the protein tag, thus preventing the formation of a covalent bond.4
Storage
Our abberior LIVE HaloX® labels are shipped freeze-dried in amounts of 10 nmol per vial. Upon arrival, the substances can be stored for up to one year at –20 °C. Dissolve the probe in DMF or DMSO shortly before staining. Once dissolved the stock solutions should be kept at –20 °C, protected from light and moisture.
Note: Depending on solvent quality the shelf-life of the stock solutions might be reduced compared to the substances in their solid form – even if stored at –20 °C.
Staining of intracellular targets using abberior LIVE HaloX® labels
The procedure described below has been successfully tested with our HaloX® probes in several stably and transiently transfected adherent mammalian cultured cell lines expressing a HaloTag® fusion protein. This procedure has yielded consistent results in most instances but may require further optimization for particular model organisms.
Required reagents; not provided
- DMF or DMSO
- glass coverslips, glass-bottom dish, or similar imaging chamber with a glass thickness of ~170 µm (No. 1.5 or No. 1.5H)
Note: We do not recommend using plastic coverslips or live-cell chambers with plastic bottoms because frequently only suboptimal imaging results are achieved. If possible, coverslips with grids, gratings, or similar should be avoided, as these structures can interfere with imaging causing aberrations that degrade image quality. - Live-cell imaging medium
- Optional: Cavity slide or coverslip holder
- Optional: silicone glue (e. g. Twinsil, Picodent)
- Fluorescence microscope with live-cell incubator, suitable excitation light source and detection filter
- Gene vector and transfection reagents
Staining procedure for cultured cells
The seeding time should be adjusted according to the doubling time of the particular cell line. Seed cells at the desired density in a cell culture chamber or onto coverslips before labeling. Use a standard cell culture medium that is optimal for the cell line. For transfection of the cells, please follow the instructions of the manufacturer of the transfection reagents.
Note: Cells grown in very high densities, i.e., a confluent layer, may give rise to a high labeling background.
For transfection of the cells, please follow the instructions of the manufacturer of the transfection reagents.
Note: Cells grown in very high densities, i.e., a confluent layer, may give rise to a high labeling background.
Staining and imaging will take place on the same day.
- Prewarm the live-cell imaging medium to the optimal temperature required to cultivate the desired cell line. In most cases this is 37 °C.
- Please dissolve the LIVE HaloX® label in DMF or DMSO to prepare a stock solution with a concentration of 1 mM.
- Prepare the staining solutions with final concentrations of 0.1 to 1 µM. For this, the stock solution of the LIVE HaloX® label is further diluted in a prewarmed live-cell imaging medium.
Note: The concentration required for proper labeling strongly depends on the specimen (tissue or cell) and can vary drastically between cell types, the expression rate of the fusion protein, and experimental conditions.
Note: Staining solutions are not stable for extended periods of time. Therefore, it is recommended to prepare only enough solution for immediate use.
Optional: The LIVE HaloX® labels can be combined with our direct LIVE labels, with our LIVE SNAP and STAR membrane probes. Simply add them to the staining solution for multicolor live-cell imaging. - Remove the cell culture medium and rinse the cells once with a prewarmed live-cell imaging medium.
- Remove the live-cell imaging medium and add a sufficient amount of staining solution to the cells. Incubate for 60 to 120 min at optimal cell growth conditions (temperature, humidity, CO2-controlled environment).
For a permanent exchange at the HaloTag® protein, probes must be present in sufficient concentration in the solution. Therefore, no washing steps must be performed. Still, the HaloX® probes are highly fluorogenic, which suppresses the background. - Embed the cells: If using cells grown on coverslips: Simply take the coverslip out of the staining solution using tweezers. Place the coverslip in a coverslip holder and fill it with the staining solution. Alternatively, mount the coverslip (cells facing downwards) onto a slide with a cavity filled with staining solution.
In case of imaging cells in a cavity slide, remove any excess staining solution using tissue paper. Gently press down the coverslip to prevent it from moving. The mounted sample can be sealed using silicone glue (e. g. Twinsil, Picodent). - After staining and mounting, the samples should be imaged directly on a microscope equipped with a live-cell incubator.
Note: For live-cell imaging, cells must always be kept at ambient conditions (temperature, humidity, pH, and CO2-conditions). This is particularly important for long-term measurements.
Abbreviations
DMF N,N-Dimethylformamide
DMSO Dimethylsulfoxide
STED Stimulated Emission Depletion
PAINT Point Accumulation in Nanoscale Topography
1 HaloX® is a registered trademark of Spirochrome AG
2 This product is covered by one or more licenses from Spirochrome AG and, is intended for Research Use Only (RUO)
3 HaloTag® is a registered trademark of Promega Corporation
4 For additional information please see the publication from J. Kompa et al., 2023, DOI: 10.1021/jacs.2c11969