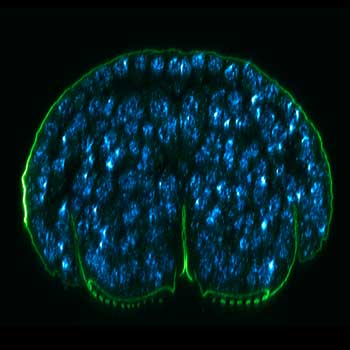
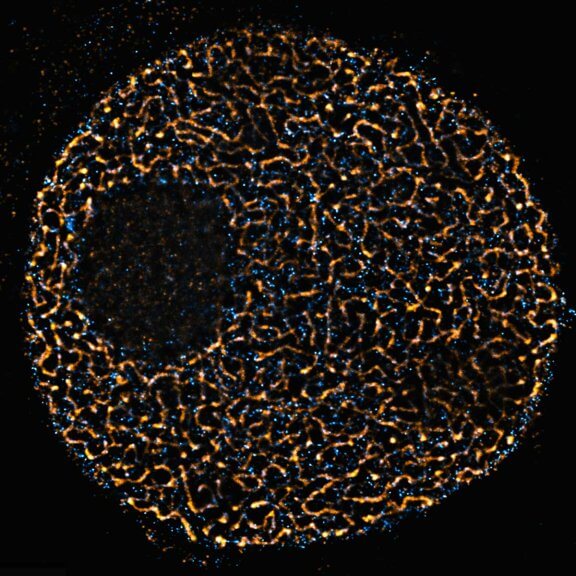
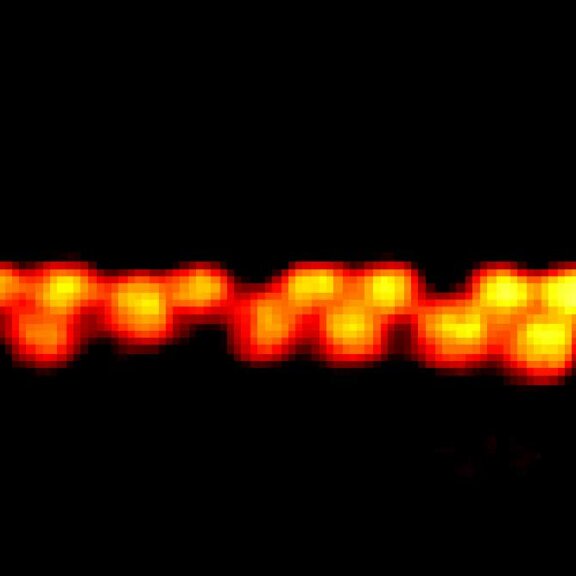
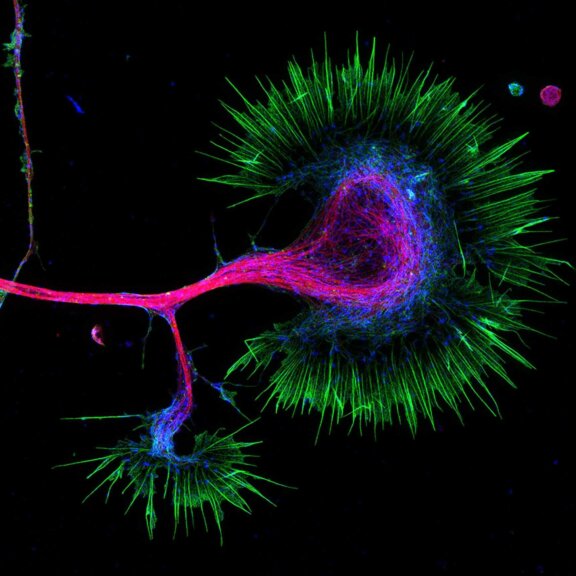
FLEXPOSURE
FLEXPOSURE adaptive illumination dramatically reduces the light dose on the sample by up to two orders of magnitude through decreasing or shutting down laser irradiation, whenever it is clear that there is no structure present at the current pixel. This substantially reduces photobleaching. Often, FLEXPOSURE makes all the difference between a crisp image and no superresolution image at all!
100% resolution, 4% light dose
MINFLUX Module
Single-digit nanometer resolution with the MINFLUX module for our MIRAVA POLYSCOPE
MATRIX Detector
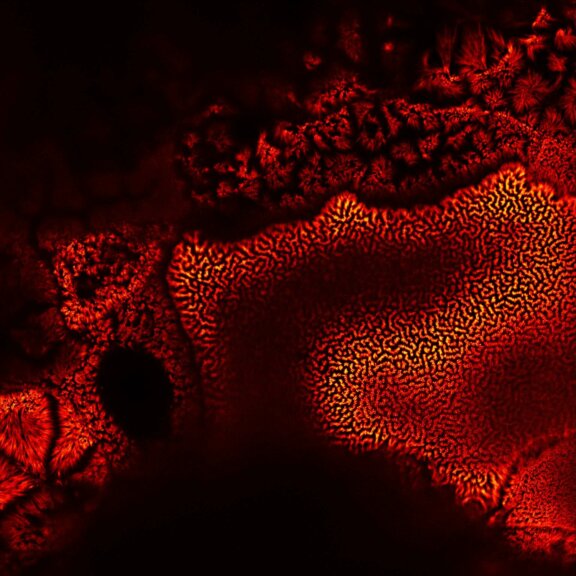
Many eyes see more than one. The MATRIX detector drastically improves signal-to-background ratio, resolution, and dynamic range.
TIMEBOW Imaging
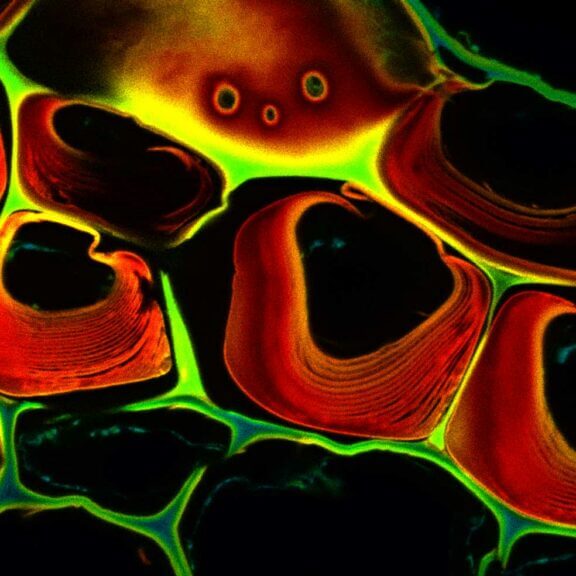
TIMEBOW lifetime imaging for stunning results at confocal and STED super-resolution.
FLEXPOSURE Illumination
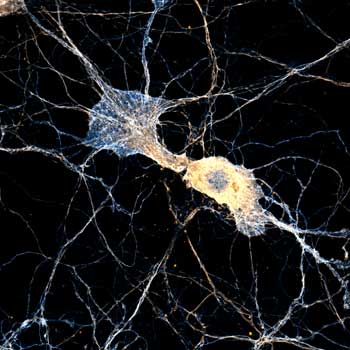
Brings down the light dose on your sample and lables dramatically. Key ingredient for volume and live-cell superresolution.
RAYSHAPE Mirror
Dynamic aberration correction with a deformable mirror over about 200 µm z-range. 140 digital actuators adjust the mirror surface within milliseconds.
Custom Solutions
We offer solutions for even the most challenging applications. Everything that can be done, we will do.
FLEXPOSURE adaptive illumination?
what it is
FAQ Video 11

“How does adaptive illumination work?”
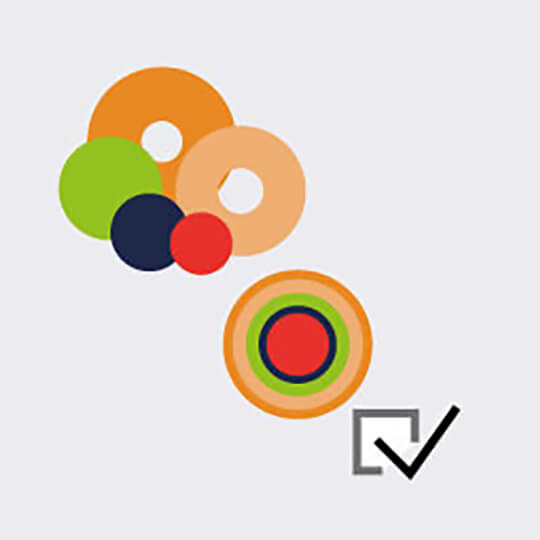
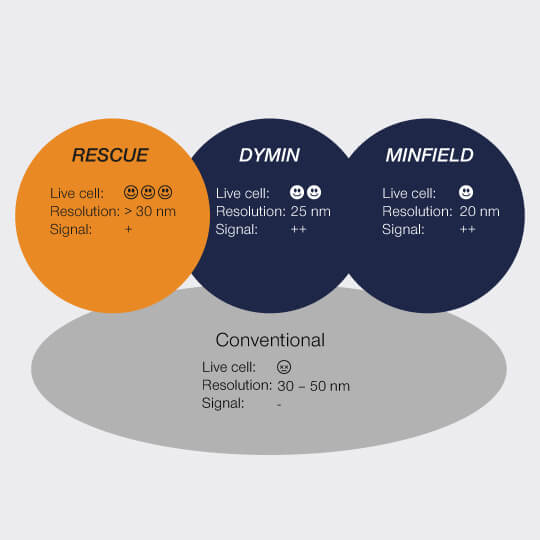
Our FLEXPOSURE adaptive illumination package consists of three related but different techniques, RESCUE, DYMIN, and MINFIELD. What they have in common is that for every pixel during the scan, they dynamically adapt the amount of laser light to the underlying structure of your sample, instead of aimlessly using the same laser power everywhere. Dark or out-of-focus areas of the image experience almost no irradiation, while regions where fluorescent markers are present and in focus, are imaged with 100% signal and resolution. This means that light is put only where it has maximal effect, and nowhere else.
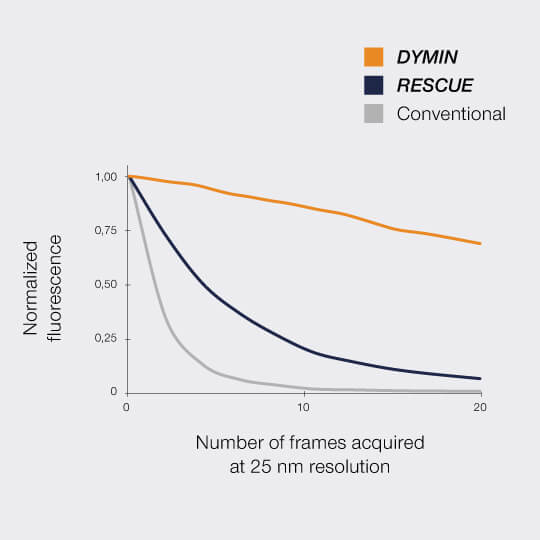
FLEXPOSURE substantially reduces photobleaching and enables long term measurements over volumes, or over dozens and dozens of frames, where conventional STED has long bleached the sample. It is the number one choice for reducing the light dose on your delicate sample!
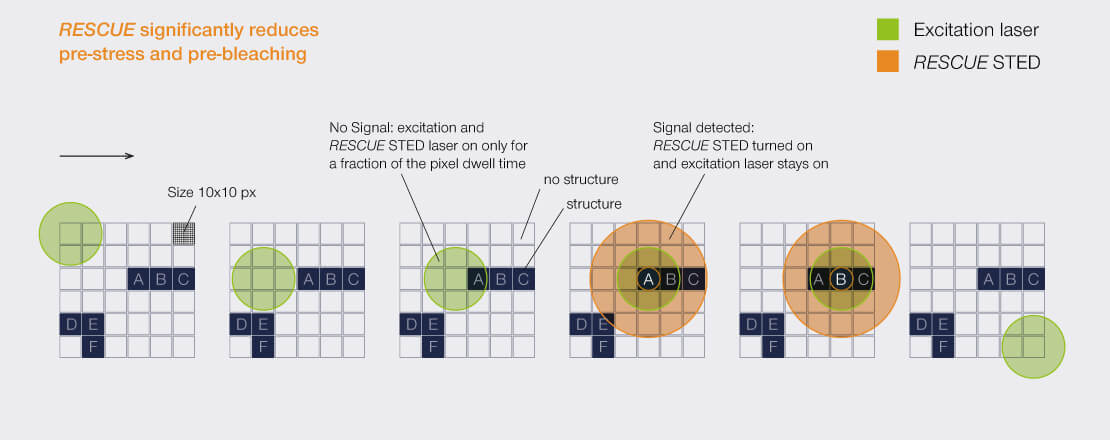
Confocal probing to the
RESCUE
For every pixel during the scan, RESCUE (Reduction of State transition Cycles) shortly probes if there is any structure and if not, illumination is immediately shut down. This quick probing is typically done in confocal mode and for a time period of about 10% of the total pixel dwell time (“confocal probing”). Only if signal is detected during this probing step, illumination and detection carries on for the remainder of the pixel dwell time.
In a typical sample, where, say, 80% of the areas are dark, RESCUE alone reduces the amount of STED photons experienced by the sample by 80%, and the amount of excitation photons by 70%, without any loss of resolution or signal whatsoever. Combine this with DYMIN and our use of pulsed instead of continuous-wave lasers, and you have a dramatic reduction in light dose down to a few percent. This really does matter for live-cell and volume imaging!
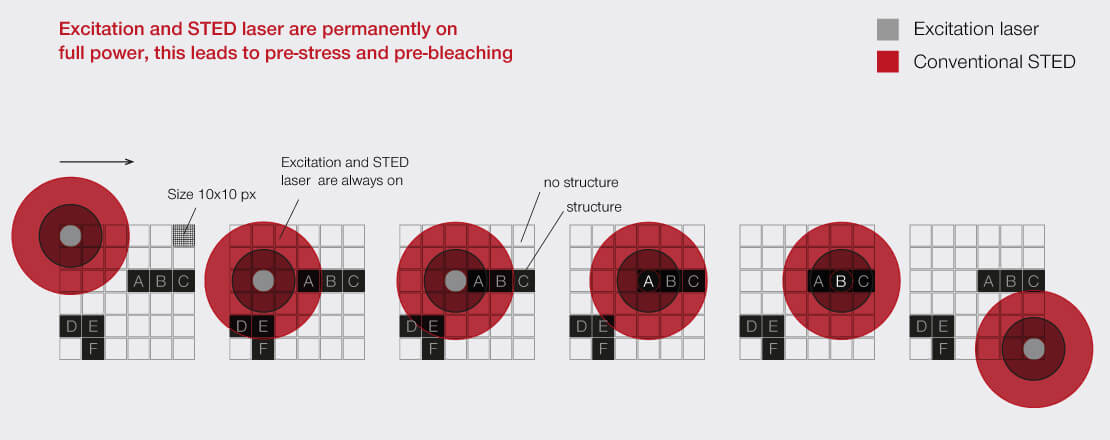
When recording a volume, i.e a stack of 2D images, the effect is even more dramatic, because the STED-donut (being diffraction limited) has a size along the z-direction that is much larger than the thickness of the individual images. Thus, recording one image means that the STED light is also applied to up to twenty others without benefit! Because of this, the gains achieved with RESCUE are amplified up to 20-fold for volume imaging.
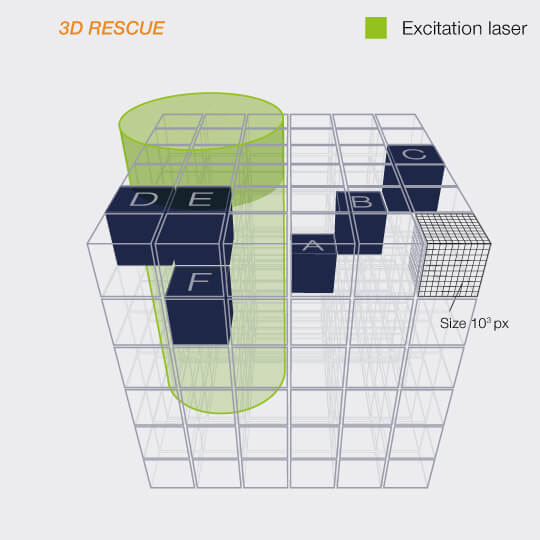
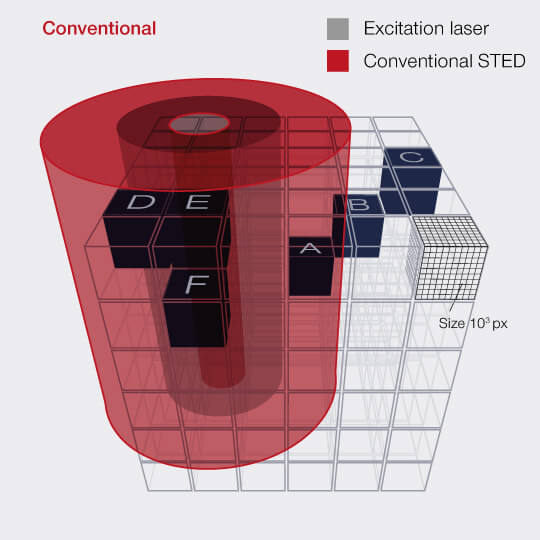
Even better probing with
DYMIN
After RESCUE, DYMIN (Dynamic Minimum) further reduces sample areas where maximum STED power is applied. This is achieved by probing for structure not only with the excitation laser, but also with a small amount of STED light, in a multi-step fashion. As with RESCUE, when no structure is detected in the first step, illumination is shut off. But if structure is present, a little bit of STED power is applied next, in order to increase the resolution, thus sharpening the probing. The trick is that small STED powers quickly yield a relatively large increase in resolution, which means that with little additional irradiation, a lot can be learned about the precise location of the fluorescent structures. The full resolution power is applied only if successive increases in STED power confirm that the scan currently targets sample structures, otherwise, light is immediately shut off for the rest of the pixel time.
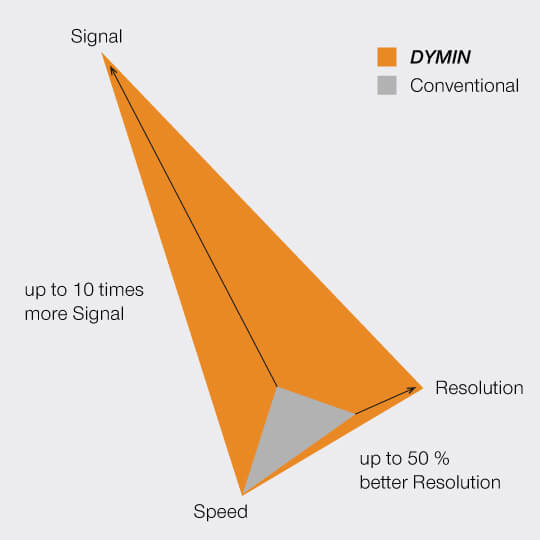
This multi-step probing at increasing resolutions reduces the overall bleaching by orders of magnitude. The gains can be invested in either dramatically more signal or significant gains in optical resolution.
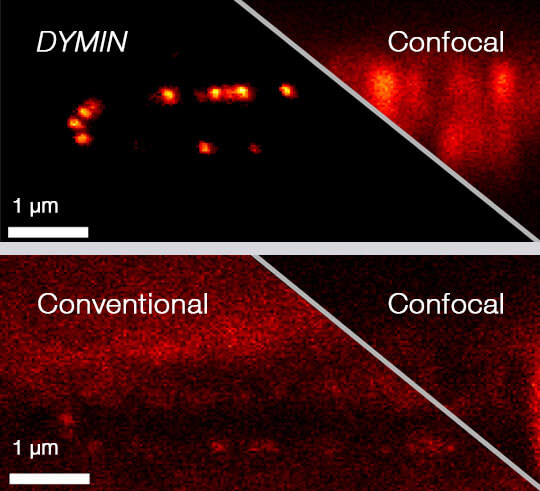
Imaging results

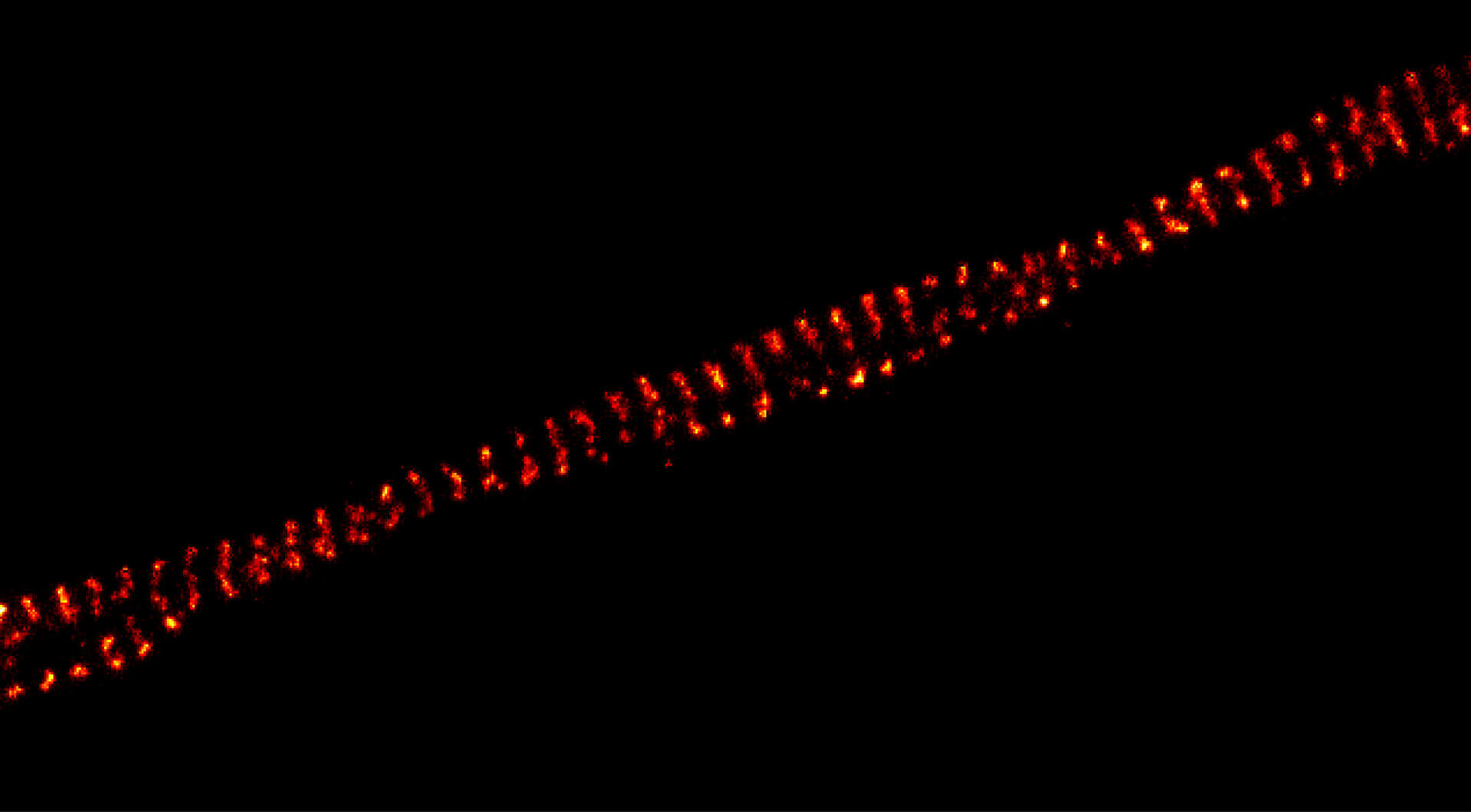
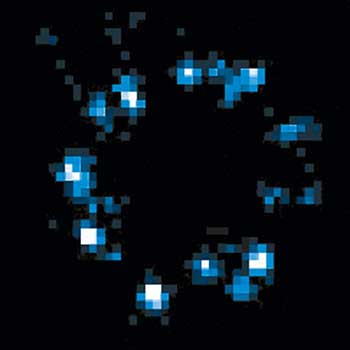
DYMIN very clearly resolves spectrin rings with an extraordinary signal-to-noise ratio. Primary hippocampal neurons (22 days in vitro) show the characteristic ~192 nm betaII spectrin periodicity along distal axons. Dye: abberior STAR 635P, Excitation: 635 nm, STED: 775 nm. Sample with courtesy from Elisa D’Este (MPIbpc).
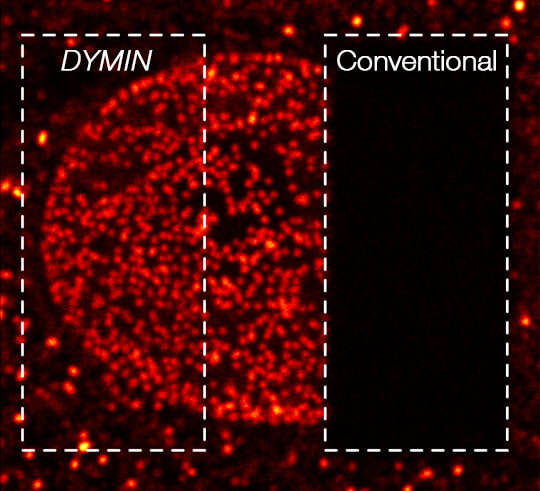
A 3D image stack of the mammalian nucleus was recorded with EASY3D and DYMIN, and conventional STED microscopy. Shown are xz sections of the stack and a confocal image after the stack-acqusition, highlighting the remarkable bleaching reduction of DYMIN. Note that DYMIN enabled us to acquire the complete stack with superior resolution and signal. Shown are Vero cells labelled with antibodies against nuclear pore complex protein (nup153).
MINFIELD
STED without STED photons
MINFIELD is yet another way to put light only where it’s needed in your sample. MINFIELD avoids scanning the STED-donut over the field of view entirely, instead only scanning the low-power central hole of the STED-donut over the fluorescent structure. Obviously, the imaged region has to be smaller than the diffraction-limited hole in the donut, but the result there is a resolution that is unmatched in the STED world.
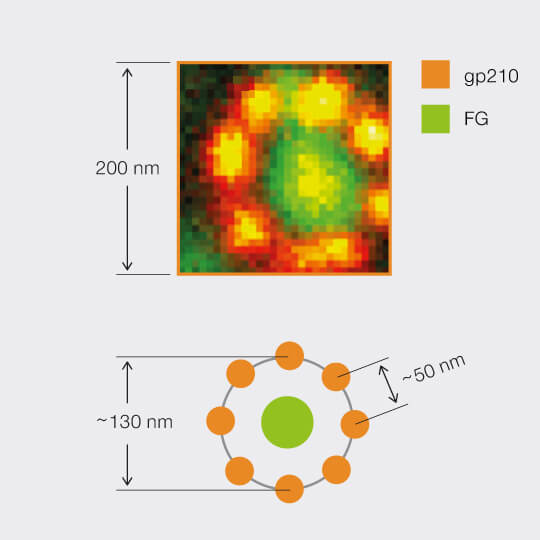
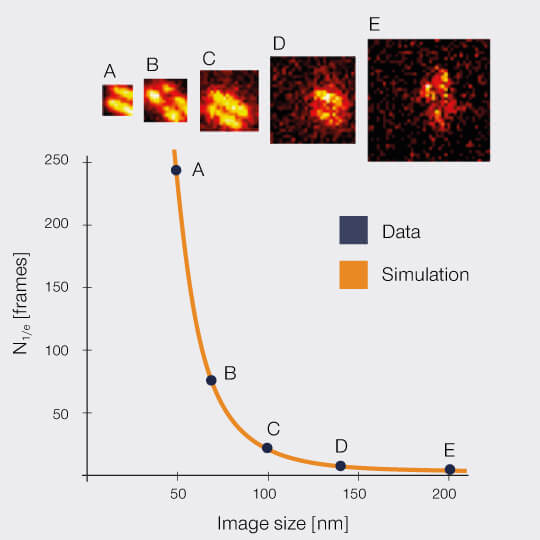
2D MINFIELD
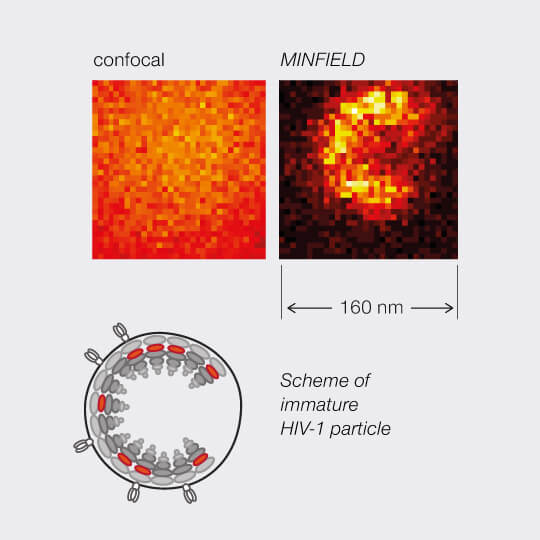
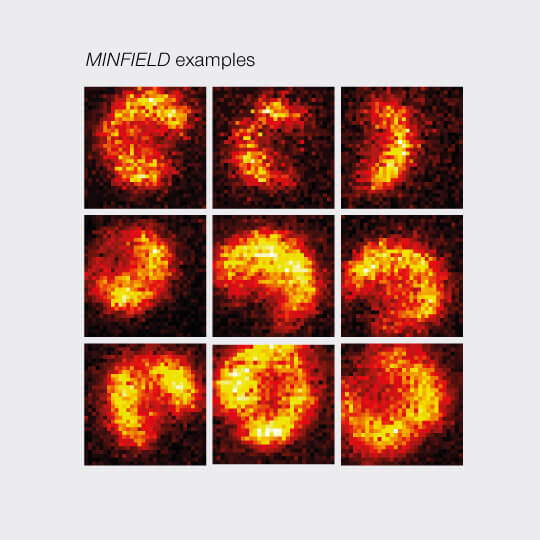
MINFIELD presented on human immunodeficiency virus type 1 (HIV-1). Confocal and MINFIELD images with a field size of 160 nm.
Images taken from J. Hanne et. al.”Stimulated Emission Depletion Nanoscopy Reveals Time-Course of Human Immunodeficiency Virus Proteolytic Maturation” ACS Nano 10, 8215-8222, 2016.
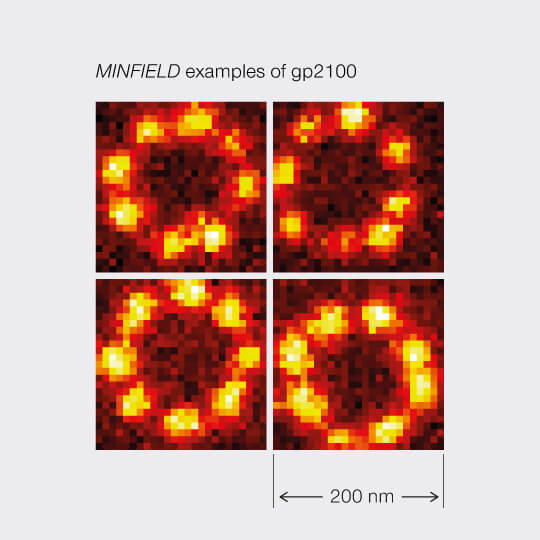

2D MINFIELD presented on gp210 and clathrin with both a MINFIELD size of 200 nm. Imaging examples of gp210 and clathrin.
3D MINFIELD
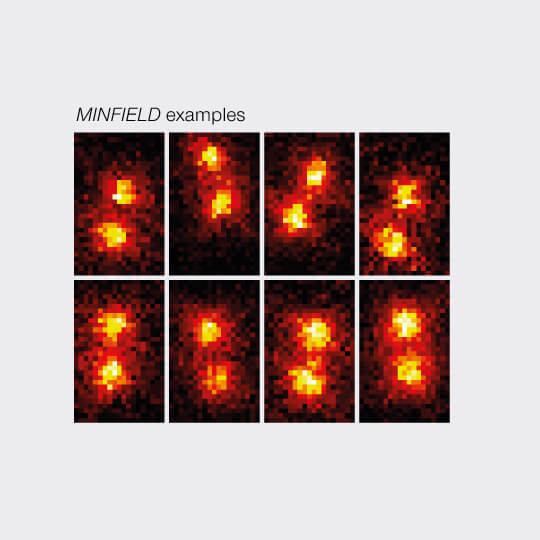
3D MINFIELD presented on standing DNA nanoruler with a nominal seperation of 91 nm, xz-view. Confocal and MINFIELD images with a field size of 180 nm x 300 nm.